SUMMARY
Falciparum malaria represents a serious and an increasing world public health problem due to the acquired parasite's resistance to the most available drugs. In some endemic areas, quinidine, a diastereoisomer of the antimalarial quinine, has been employed for replacing the latter. In order to evaluate the use of quinidine as an alternative to the increasing loss of quinine effectiveness in Brazilian P. falciparum strains, as has been observed in the Amazon area, we have assayed quinidine, quinine and chloroquine. The in vitro microtechnique was employed. All isolates showed to be highly resistant to chloroquine. Resistance to quinine was not noted although high MIC (minimal inhibitory concentration) values have been observed. These data corroborate the decreasing sensitivity to quinine in strains from Brazil. Quinidine showed IC^sub 50^ from 0.053 to 4.577 (mu)mol/L of blood while IC^sub 50^ from 0.053 to 8.132 (mu)mol/ L of blood was estimated for quinine. Moreover, clearance of the parasitemia was observed in concentrations lower than that used for quinidine in antiarrhythmic therapy, confirming our previous data. The results were similar to African isolate.
KEYWORDS: Quinidine; Malaria; Quinine; Chloroquine; P. falciparum; Antimalarial resistance
INTRODUCTION
Current malaria chemotherapy was considered to start in the middle of the XVII century with the jesuit's discovery of the folkloric use of Cinchona bark as a febrifuge by South American indians. However, Artemisia annua was already known by Chinese population since the beginning of the Christian era13, although the pharmacological studies have started in the 70's .
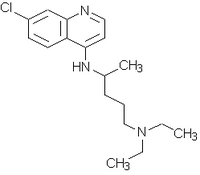
After its introduction in Europe, Cinchona tree bark powder begun to be extensively employed in the treatment of fevers. In 1820, two alkaloids, quinine (Fig. 1) and cinchonine, were isolated but the antimalarial effect was attributed only to quinine. The pure alkaloid replaced the Cinchona bark and quinine became the only drug in the therapy and prophylaxis of malaria38.
The unavailability of quinine during World Wars I and II led to the advent of the synthetic drugs, from which chloroquine stood out. Considered to be the ideal antimalarial, chloroquine showed to be less toxic, more effective and better to be administered than quinine, therefore, substituting it36.
Plasmodium falciparum strains resistance to chloroquine and to the second-line therapy sulfadoxine-pyrimethamine since the 60's led to the re-introduction of quinine. This alkaloid has been specially used to treat severe or complicated infections and chloroquine or mefloquine resistant malaria. In the later ones, the combination therapy has been recommended for maintaining the antimalarial effectiveness. For instance, tetracycline has been used in combinations with quinine40. Quinine resistant strains were identified in the early stages of the drug usage. Lately, a continuous decreasing in parasite sensitivity has been noted along different endemic areas8,24,27. This has increased the interest in using quinidine (Fig. 1), the dextro-rotatory diastereoisomer of quinine, as an alternative therapy.
Quinidine had its antimalarial activity despised in function of quinine. Recognized as a potent antiarrhythmic agent, quinidine has been mostly employed in the cardiovascular therapy since the 20's29. Nevertheless, in vitro, in vivo and clinical studies have reported quinidine as an effective or even superior to quinine as an antimalarial agent5,7,11,21,22,24,25,32,33,39. For a long time, quinidine has been considered by the Centers for Disease Control (CDC/Drug Service) in USA14,15 to be the drug of choice in the treatment of complicated P. falciparum infection. Since the 80's it has shown to be useful for treating pregnant women with severe falciparum malaria25, since abortifacient properties are alleged to quinine40. Moreover, quinidine, available worldwide, has increasingly been the only useful therapy for severe and complicated malaria since USA stopped the manufacture of quinine25.
With the purpose of facing the challenge of quinine effectiveness decrease in Brazilian P falciparum strains1,2,8,9,16,26,34,41, we evaluated the in vitro sensitivity of isolates from North Brazilian States to quinidine in comparison to quinine and chloroquine. We were based on previous studies when quinidine showed to exert antimalarial effect in concentrations many times inferior than those used in the cardiovascular therapy23.
MATERIAL AND METHODS
Drugs - The drugs used were quinidine bisulphate dihydrate (Asta Medica Ltda), quinine sulphate (Central de Medicamentos, CEME) and chloroquine diphosphate (Fundacao para o Remedio Popular, Furp).
Isolates - Six isolates of P falciparum were employed: Isolate 1 -- 5,760 parasites/mm^sup 3^; Isolate 2 - 65,280 parasites/mm^sup 3^; Isolate 3 - 3,240 parasiteS/mm^sup 3^ and Isolate 4 - 65,000 parasites/mm^sup 3^. All these isolates were assayed immediately after blood venum puncture. Isolates 1-3 were obtained from patients infected in Brazilian Northern region and Isolate 4 from a patient infected in Angola. These people had not been submitted to any antimalarial treatment for the previous 28 days10. Blood samples were collected after formal consent from patients. Isolate 5 (SUCEN S20-87) was obtained from a patient infected in Rondonia, Brazil, in 1987. Isolate 6 (Uganda Palo Alto) was used as a reference for chloroquine sensitivity. Isolates 5 and 6 were assayed after synchronization of 10% ring forms parasitemia.
Drug sensitivity assay - The biological assay was the in vitro microtechnique28. 96 flat-bottom wells microplates contained quinidine (at the same concentrations range than quinine), quinine and chloroquine were titrated (Table 1). Each series included the usual antimalarial therapeutic concentrations of quinine (1.14 (mu)mol/L of blood) and chloroquine (6.4 (mu)mol/L of blood) as the medium of the twofold serial dilutions. This procedure was adopted in analogy to the test for the assessment of P. falciparum response to antimalarial drugs as stated by WHO10.
The infected blood was diluted in culture medium to a 10% haematocrit. The plates were incubated at 37 degC for 24-48 hours (depending on schizont maturation) using the candle jar method37. The number of schizonts with three or more nuclei was determined in 200 parasites in thick blood smear stained with Giemsa.
Schizonts growth at 1.6 (mu)mol/L of blood for chloroquine and 51.2 (mu)mol/L of blood for quinine were considered as threshold for in vitro P. falciparum resistance24. The minimal inhibitory concentration (MIC), defined as the lowest concentration that completely inhibited schizont formation, was determined.
Statistical analysis - The parasitemia rate (number of parasites in the different wells in relation to the control) in function of the concentration was analyzed throughout the linear logistic model17 for quinine and quinidine. According to this, coincident, parallel, concurrent (1 intercept) and concurrent (2 intercepts) lines were fitted. The comparisons were made with regard to the simplest model, coincident lines. The goodness-of-fit statistic and the corresponding p-value were computed to assess the adequacy of the model. A significance level of 10% was adopted. Then, the medium inhibitory concentration (IC^sub 50^) and the correspondent confidence interval (95%) were estimated for each drug.
RESULTS
The in vitro sensitivity of P falciparum isolates to quinidine and the classical antimalarials quinine and chloroquine is shown in Table 1. Based on MIC, all parasites showed to be resistant to chloroquine, except the Isolate 6. Different susceptibility levels were observed to quinidine and quinine. No resistance to quinine was detected.
The results of the statistical analysis are depicted in Tables 2 and 3 and in Figure 2. For Isolate 1 the best-selected model was the concurrent lines (1 intercept). As the concentration increases, the parasitemia rate decrease is higher for quinidine than for quinine, leading to a lower quinidine IC^sub 50^ (Table 3). For Isolate 2 parallel lines was the best-fitted model, with the quinine parasitemia rate and consequent IC^sub 50^ smaller than those of quinidine. Even though none of the proposed models was well fitted for Isolate 3, the concurrent lines model (2 intercepts) indicates that for small and moderate concentrations quinine has a smaller parasitemia rate while an opposite tendency appeares in larger concentrations. Equal IC^sub 50^ values were estimated. For Isolate 4, the concurrent lines model (2 intercepts) was the selected model. From the fitted lines, small concentrations of quinidine cause lower parasitemia rates while an opposite behavior is observed in high concentrations. Quinidine at 50% inhibition showed to be a worse antiplasmodial agent than quinine. For Isolate 5, coincident lines was the selected model and a common fitted line as well as estimated IC, values were obtained. For Isolate 6, concurrent lines (1 intercept) was the best-fitted model. In this case, the parasitemia rate of quinine was smaller than the quinidine and the difference increases with the concentration. These results corroborate the estimated IC^sub 50^ values (Table 3).
DISCUSSION
The loss in P. falciparum sensitivity to quinine in Brazil dated from the beginning of the XX century. A continuous decrease has been reported in special since the 80's when quinine replaced chloroquine and sulfadoxine-pyrimethamine in malaria resistant therapy. Then, tetracycline began to be co-administered with quinine in order to overcome the resistance problem and to reduce its inherent toxicity2,8,9. Quinine is still considered a useful drug for treating non-complicated falciparum malaria8. Nevertheless, a gradual increase in the time to clear the parasitemia followed by an elevation in recurrence frequency were observed in patients from the Eastern Amazon, where quinine was employed during the quadriennial 1983-199426. Moreover, decrease in quinine sensitivity was also evidenced in two recent reports performed with Brazilian samples from Mato Grosso State, Amazon area16,41.
In our study, different susceptibility levels were observed among the isolates in relation to quinine and quinidine. Resistance to quinine was not detected since no schizont maturation was observed at 51.2 (mu)mol/L of blood24. Nevertheless, decreasing in P. falciparum susceptibility to quinine was confirmed, in particular, with Isolate 1 (the highest IC^sub 50^) and with Isolates 5 and 6, collected for a long time and much more sensitive to quinine. These data corroborated recent evaluations16,26,41, showing a unquestionable and worryingly loss of quinine effectiveness in Brazilian strains. Interestingly, quinine was more effective against the African isolate, Isolate 4. However, opposite data have been obtained with isolates from Gabon24 and Niger35 and, also, out of the African continent, in Thailand32. In addition, all isolates showed to be highly resistant to chloroquine. MIC values were much higher than the considered threshold concentration, 1.6 (mu)mol/L of blood24.
We consider that as observed in former studies 5,7,11,21,22,24,25,32,33,39, quinidine shows a promising activity against Brazilian P. falciparum strains. Quinidine inhibitory effect was observed in concentrations lower (estimated IC^sub 50^ ranged from 0.053 to 4.577 (mu)mol/L of blood that corresponds to 0.0172 to 1.485 (mu)g/mL) than those employed in antiarrhythmic therapy, 2-6 (mu)g/mL4. These results are in accordance to our previous assay carried out with two North Brazilian P. falciparum isolates23. Although further in vitro, in vivo, and clinical studies should be conducted, the preliminary results here reported are indicative of the possibility of using quinidine in uncomplicated but, especially, in complicated and severe forms of falciparum malaria. This finding assumes greater importance with the increasing loss of quinine efficacy against Brazilian strains. Nevertheless, precautions have been recommended concerning to the intravenous dispensing of quinidine due to its inherent cardiotoxicity6,25,40. In addition, the exchange transfusion after quinidine administration has been recommended for rapidly reducing the parasitemia, lowering the mortality by severe falciparum malaria20.
Notwithstanding, quinidine may also be used in combination with other antimalarial agents as well as with its stereoisomers. Cinchona alkaloid combinations have been successfully used for treating falciparum malaria in endemic areas3,12,18.19,31. Quinidine, quinine and cinchonine (Quinimax(R)) or even quinidine, quinine, cinchonine and chinchonidine have been employed by different administration routes for treating uncomplicated as well as complicated falciparum malaria. These combination regimens allow to reduce the individual doses of the alkaloids, decreasing their inherent toxicities.
RESUMO
Avaliacao da sensibilidade in vitro ak quinidina em isolados brasileiros de Plasmodium falciparum: analise comparativa A quinina e a cloroquina
Malaria falciparum representa grave e crescente problema de saude publica mundial, dada a resistencia do parasito a maioria dos farmacos disponfveis. Em algumas areas endemicas, a quinidina, diastereoisomero do antimalarico quinina, vem sendo empregada em substituicao a este ultimo. Com o objetivo de avaliar o emprego da quinidina como alternativa A perda crescente de sensibilidade de cepas brasileiras de P. falciparum a quinina, como o observado na regiao Amazonica, realizamos ensaio comparativo entre quinidina, quinina e cloroquina. A tecnica in vitro do microteste de sensibilidade foi utilizada. Todos os isolados mostraram-se altamente resistentes A cloroquina. Resistencia a quinina nao foi observada, embora altos valores de CMI (concentracao minima inibitoria) tenham sido encontrados. Estes resultados corroboram o decrescimo de suscetibilidade de cepas brasileiras a quinina. Observouse variacao de IC^sub 50^ de 0,053 a 4,577 (mu)mol/L de sangue para a quinidina, enquanto para a quinina estimou-se IC^sub 50^ de 0,053 a 8,132 (mu)mol/L de sangue. Ademais, observou-se clareamento da parasitemia em concentracoes inferiores A da quinidina quando empregada como farmaco antiarritmico, confirmando estudo anterior por nos realizado. Resultados semelhantes foram encontrados em isolado oriundo da Africa.
REFERENCES
1. ALECRIM, M.G. - Resistance to in vivo and in vitro chemotherapies in the Brazilian Amazonia. Mem. Inst. Oswaldo Cruz, 81(suppl. 2): 153-157, 1986.
2. BARATA, L.C.B.; BOULOS, M. & DUTRA, A.P. - Emprego da associa:lo tetraciclina e quinino no tratamento da malaria causada pelo Plasmodium falciparum. Rev. Soc. bras. Med. trop., 19: 135-137, 1986.
3. BARENNES, H.; MUNJAKAZI, J.; VERDIER, F.; CLAVIER, F. & PUSSARD, E. - An open randomized clinical study of intrarectal versus infused Quinimax(R) for the treatment of childhood cerebral malaria in Niger. Trans. roy. Soc. trop. Med. Hyg., 92: 437-- 440, 1998.
4. BENET, L.Z.; OIE, S. & SCHWARTZ, J.B. - Design and optimization of dosage regimens: pharmacokinetic data. In: HARDMAN, J.G.; LIMBIRD, L.E.; MOLINOFF, P.B.; RUDDON, R.W. & GILMAN, A.G., ed. Goodman & Gilman's: the pharmacological basis of therapeutics. 9. ed. New York, Pergamon-Press, 1996. p. 1777.
5. BHASIN, VK. & TRAGER, W. - Gametocytocidal effect in vitro of cinchona alkaloids and derivatives on a Plasmodium falciparum clone. Acta leidensia, 55: 151-158, 1987.
6. BHAVNANI, S.M. & PRESTON, S.L. - Monitoring of intravenous quinidine infusion in the treatment of Plasmodium falciparum malaria. Ann. Pharmacother., 29: 33-35, 1995.
7. BJORKMAN, A.; WILLCOX, M.; MARBIAH, N. & PAYNE, D. - Susceptibility of Plasmodium falciparum to different doses of quinine in vivo and to quinine and quinidine in vitro in relation to chloroquine in Liberia. Bull. Wid. Hlth. Org., 69: 459-465, 1991.
8. BOULOS, M.; DUTRA, A.P.; DI SANTI, S.M.; SHIROMA, M. & AMATO NETO, V. -- Avaliacao clinica do quinino para o tratamento de malaria por Plasmodium falciparum. Rev. Soc. bras. Med. trop., 30: 211-213, 1997.
9. BOULOS, M.; DI SANTI, S.M.; BARATA, L.C.B. et al. - Some aspects of treament, prophylaxis and chemoresistance of Plasmodium falciparum malaria. Mem. Inst. Oswaldo Cruz, 81(suppl. 2): 255-257, 1986.
10. BRUCE-CHWATT L.J. - Chemotherapy of malaria. In: BRUCE-CHWA`17, L.J., ed. Chemotherapy of malaria. 2. ed. Geneva, World Health Organization, 1982. p. 211-223.
11. BUNNAG, D.; HARINASUTA, T.; VANIJANONTA, S. et al. - Slow-release quinidine in the treatment of chloroquine resistant falciparum malaria: a double-blind trial. Acta leidensia, 55: 129-138, 1987.
12. BUNNAG, D.; HARINASUTA, T; VANIJANONTA, S. et al. - Treatment of chloroquine-- resistant falciparum mall aria with a combination of qu in ine, qu in i di ne and cinch o nine (LA40221) in adults by oral and intravenous administration. Acta leidensia, 55: 139-149, 1987.
13. BUSS, A.D. & WAIGH, R.D. - Antiparasitic drugs. In: WOLFF, M.E., ed. Burger's medicinal chemistry and drug discovery. 5. ed. New York, Wiley-Interscience, 1995. v. 1, p. 1021-1028.
14. CENTERS FOR DISEASE CONTROL AND PREVENTION (CDC) - Treatment with quinidine gluconate of persons with severe Plasmodium falciparum infection: discontinuation of parenteral quinine from CDC Drug Service. M.M.W.R., 40(RR4): 21-23,1991.
15. CENTERS FOR DISEASE CONTROL AND PREVENTION (CDC) - Treatment of severe Plasmodium falciparum malaria with quinidine gluconate: discontinuation of parenteral quinine from CDC drug service. M.M.W.R., 40(14): 240, 1991.
16. CERU771 JR., C.; MARQUES, C.; DE ALENCAR, F.E.C. et al. - Antimalarial drug susceptibility testing of Plasimodium falciparum in Brazil using a radioisotope method. Mem. Inst Oswaldo Cruz, 94: 803-809, 1999.
17. COLLETT, D. - Modelling binary data. London, Chapman & Hall, 1991.
18. DELORON, P.; LEPERS, LP.; VERDIER, F. et al. - Efficacy of a 3-day oral regimen of a quinine-quinidine-cinchonine association (Quinimax(R)) for treatment of falciparum malaria in Madagascar. Trans. roy. Soc. trop. Med. Hyg., 83: 751-754, 1989.
19. DRUILHE, P; BRANDICOURT, O.; CHONGSUPHAJAISIDDHI, T. & BERTHS, J. Activity of a combination of three cinchona bark alkaloids against Plasmodium falciparum in vitro. Antimicrob. Agents Chemother., 32: 250-254, 1988.
20. JOTTE, R.S. & SCOTT, J. - Malaria: review of features pertinent to emergency physician. J. Emerg. Med., 11: 729-736, 1993.
21. KAZIM, M.; PURI, S.K. & DUTTA, G.P. - Comparative evaluation of blood schizontocidal activity of quinine and quinidine against drug resistant rodent malaria. J. common. Dis., 23: 254-256,1991.
22. KILIMALI, V.A. - The in vitro response of Plasmodium falciparum to amodiaquine, quinine and quinidine in Tanga region, Tanzania. E. Afr. med. J., 67: 336-340, 1990. 23. MENEZES, C.M.S. - Estudo da influencia das propriedades fisico-quimicas na
reversao da resistencia do Plasmodium falciparum it cloroquina. Sin Paulo, 1997. (Tese de Doutoramento - Faculdade de Ciencias Farmaceuticas da Universidade de Sdo Paulo).
24. PHILIPPS, J.; RADLOFF, P.D.; WERNSDORFER, W. & KREMSNER, PG. - Followup of the susceptibility of Plasmodium falciparum to antimalarials in Gabon. Amer. J. trop. Med. Hyg., 58: 612-618, 1998.
25. PHILLIPS, R.E.; WARRELL, D.A.; WHITE, N.J.; LOOAREESUWAN, S. & KARBWANG, J. - Intravenous quinidine for the treatment of severe falciparum malaria. Clinical and pharmacokinetic studies. New Eng. J. Med., 312: 1273-1278, 1985.
26. PINELI, L.L.; SCHOEPFER, A.C.A.; JARDIM, D.V; SANTOS, E.R. & ALMEIDA NETO, J.C. - MalAria por Plasmodium falciparum. Analise quadrienal, durante 12 anos, da eficAcia do tratamento com quinino. Rev. Soc. bras. Med. trop., 32: 241245, 1999.
27. PUKRITTAYAKAMEE, S.; SUPANARANOND, W.; LOOAREESUWAN, S.; VANIJANONTA, S. & WHITE, N.J. - Quinine in severe falciparum malaria: evidence of declining efficacy in Thailand. Trans. roy. Soc. trop. Med. Hyg., 88: 324-327, 1994.
28. RIECKMANN, K.H.; CAMPBELL, G.H.; SAX, L.J. & MREMA, J.E. - Drug sensitivity of Plasmodium falciparum. An in vitro microtechnique. Lancet, 1: 22-23, 1978.
29. RODEN, D.M. - Antiarrhythmic drugs. In: HARDMAN, J.G.; LIMBIRD, L.E.; MOLINOFF, P.B.; RUDDON, R.W. & GILMAN, A.G., ed. Goodman & Gilman's: the pharmacological basis of therapeutics. 9. ed. New York, Pergamon-Press, 1996. p. 869-870.
30. ROGIER, C.; BRAU, R.; TALL, A.; CISSE, B. & TRAPE, J.F. - Reducing the oral quinine-quinidine-cinchonin (Quinimax(R))') treatment of uncomplicated malaria to three days does not increase the recurrence of attacks among children living in a highly endemic area of Senegal. Trans. roy. Soc. trop. Med. Hyg., 90: 175-178, 1996.
31. SABCHAREON, A.; CHONGSUPHAJAISIDDHI, T.; ATTANATH, P. er al. - Red cell and plasma concentrations of combined quinine-quinidine and quinine in falciparum malaria. Ann. trop. Paediat., 11: 315-324,1991.
32. SABCHAREON, A.; CHONGSUPHAJAISIDDHI, T.; SINHASIVANON, V.; CHANTHAVANICH, P. & ATTANATH, P. -In vivo and in vitro responses to quinine and quinidine of Plasmodium falciparum. Bull. Wid. HIM Org., 66: 347-352,1988.
33. SANDERS, J.P. - Treatment of malaria with a short course of quinidine. Amer. J. trop. Med. Hyg., 15: 651-660, 1935.
34. SEGURADO, A.A.C.; DI SANTI, S.M. & SHIROMA, M. - In vivo and in vitro Plasmodium falciparum resistance to chloroquine, amodiaquine and quinine in the Brazilian Amazon. Rev. Inst. Med. trop. S. Paulo, 39: 85-89, 1997.
35. SOWUNMI, A.; SALAKO, L.A.; LAOYE, O.J. & ADEROUNMU, AX. - Combination of quinine, quinidine and cinchonine for the treatment of acute falciparum malaria: correlation with the susceptibility of Plasmodium falciparum to the cinchona alkaloids in vitro. Trans. roy. Soc. trop. Med. Hyg., 84: 626-629, 1990.
36. TRACY, JX & WEBSTER JR., L.T. - Drugs used in the chemotherapy of protozoa infections: malaria. In: HARDMAN, J.G.; LIMBIRD, L.E.; MOLINOFF, P.B.; RUDDON, R.W. & GILMAN, A.G., ed. Goodman & Gilman's: the pharmacological basis of therapeutics. 9. ed. New York, Pergamon Press, 1996. p. 965-985.
37. TRAGGER, W. & JENSEN, B.B. - Human malaria parasites in continuous culture. Science, 193: 673-675, 1976.
38. WERNSDORFER, W.H. - The importance of malaria in the world. In: KREIER, J.P., ed. Malaria. New York, Academic Press, 1980. v. 1, p. 1-79.
39. WHITE, N.J.; LOOAREESUWAN, S.: WARRELL, D.A. et al. - Quinine in falciparum malaria. Lancet, 2: 1069-1071, 1981.
40. WORLD HEALTH ORGANIZATION (WHO) - WHO model prescribing information. Drugs used in parasitic diseases. 2. ed. Geneva, World Health Organization, 1995. p. 24-54.
41. ZALIS, M.G.; PANG, L.: SILVEIRA, M.S.: MILHOUS, W.K. & WIRTH, D.F. -- Characterization of Plasmodium falciparum isolated from the Amazon region of Brazil: evidence for quinine resistance. Amer. J. trop. Med. Hyg., 58: 630-637. 1998.
Received: 17 August 2000 Accepted: 16 March 2001
Carla M. S. MENEZES(1), Karin KIRCHGATTER(2), Silvia Maria DI SANTI(2), Gilberto A. PAULA(3) & Elizabeth I. FERREIRA(1)
(1) Faculdade de Ciencias Farmaceuticas, Universidade de Sao Paulo, Sao Paulo, SP, Brasil.
(2) Divisao de Programas Especiais, Superintendencia de Controle de Endemias (SUCEN), Sao Paulo, SP, Brasil.
(3) Instituto de Matematica e Estatistica, Universidade de Sao Paulo, Sao Paulo, SP, Brasil.
Correspondence to: Dr Elizabeth Igne Ferreira, Faculdade de Ciencias Farmaceuticas/USP, Av. Prof. Lineu Prestes 580, B1 13, 05508-900 Sao Paulo, SP, Brasil. e-mail: hajudan@usp.br
Copyright Instituto de Medicina Tropical de Sao Paulo Jul/Aug 2001
Provided by ProQuest Information and Learning Company. All rights Reserved