Topical antiseptics are essential for infection control. Antiseptic formulations employ a variety of mechanisms, act at various rates and persistence intervals, demonstrate various levels of toxicity, and are more or less likely to trigger resistance. The desired characteristics are considered when selecting antiseptics for hand washing, surgical scrubbing, and patient preoperative skin preparation. The selection process requires evidence of product safety and efficacy. This article explores currently available topical antimicrobial agents used in medical settings.
ABBREVIATIONS: CHG = chlorhexidine gluconate; FDA = Food and Drug Administration; FR = Federal Register; GRASE = generally recognized as safe and effective; MRSA = methicillin resistant Staphylococcus aureus; NDA = new drug application; OTC = over-the-counter; PCMX = chloroxylenol; RASE = recognized as safe and effective; TFM = tentative final monograph; USP = United States Pharmacopeia.
INDEX TERMS: antiseptics; healthcare antiseptic; topical antiseptic.
Clin Lab Sci 2005;18(3):160
LEARNING OBJECTIVES
1. Define transient flora and resident flora and compare the two groups in terms of ease of removal.
2. Describe the FDA regulatory approval process for a drug product.
3. Define the following terms: antimicrobial soap, skin antiseptic, Healthcare personnel handwash, surgical hand scrub, and patient preoperative skin preparation.
4. Identify the types of healthcare topical antiseptic currently available on the market.
5. Identify factors that may influence the antimicrobial action of an antiseptic.
GLOSSARY OF TERMS
* Advisory Review Panels are composed of scientifically qualified members and nonvoting technical liaison members representing consumer and industry interests. These panels are charged with reviewing the ingredients and labeling of marketed OTC drug products to determine whether they could be classified as GRASE for use in self-treatment.
* Food and Drug Administration or the FDA is a federal agency in the Department of Health and Human Services established to regulate the release of new foods, drugs, and health-related products.
* GRASE is a term used to describe nonprescription drug products that are generally recognized as safe and effective.
* Misbranded is to label a drug product falsely or in a misleading way.
* Monograph is a list of therapeutic classes of ingredients that are generally recognized as safe and effective. A manufacturer wanting to market a product containing an ingredient covered under the OTC monograph need not seek the FDA's prior approval.
* NDA is a new drug application which requires that the drugs be proven safe and effective for human use before being marketed.
* OTC drugs are nonprescription drugs that are considered to be safe and effective for consumers to use without professional supervision, provided the required label directions and warnings are followed.
* RASE is a term used to describe prescription drug products that are recognized as safe and effective.
* TFM or tentative final monograph represents FDA's tentative conclusions as a proposed rule. This document offers the first clear signal of FDA's ultimate intentions. After the TFM is published, a period of time is allotted for objections or requests for a public hearing. New data may be submitted during this period.
INTRODUCTION
Topical antiseptics are antimicrobial agents that kill, inhibit, or reduce the number of microorganisms on the skin.1 The human skin is colonized by a wide variety of microorganisms that may provide a protective mechanism to the host but also serve as a source of infection. Organisms that do not cause disease are called the "usual or normal flora". Normal flora on the skin are "transient" or "resident". Transient flora are contracted from the environment or from other people. In most cases, these organisms are not part of the established normal flora.2 Healthcare professionals, for example, acquire microbes including methicillin-resistant Staphylococcus aureus (MRSA) during contact with patients or contaminated surfaces. Although transient organisms are easily removed from the upper layer of the skin along with dirt particles and oil, they may become part of the resident established flora of individuals. Resident flora can be persistently isolated from the hands of most people. These organisms include coagulase-negative staphylococci, Corynebacterium (diptheroids or coryneforms), Pmprionibacterium, and Acinetobacter species.
Topical antiseptics are active against both resident and transient flora on intact skin and are able to reduce microbial numbers on the skin by mechanical removal, chemical action, or both. When selecting an antiseptic for healthcare professionals, the following considerations should be taken into account: efficacy, maximal killing of both transient and resident bacteria, rapidity of antimicrobial action, persistence of activity, ease of use, and lack of skin irritation.3,4
There are many types of topical antiseptics designed for various purposes; each may be used for healthcare, veterinary workers, food-handlers, or public consumers. Topical antimicrobials are considered drugs by the FDA and are regulated as such. This article provides an overview on antiseptics and discusses factors influencing their activity, mechanisms of action, safety, and toxicity. A brief history of the regulatory approval process of these products is also described. The Healthcare Infection Control Practices Advisory Committee provides an in-depth review and guideline for hand antisepsis in healthcare settings.4
REGULATORY HISTORY OF TOPICAL ANTISEPTICS
On September 13, 1974, the FDA published an advance notice of proposed rulemaking in the Federal Register (FR) to establish a monograph for over-the-counter (OTC) topical antimicrobial drug products. The notice incorporated the recommendations of the Advisory Review Panel on OTC Topical Antimicrobial Drug Products (Antimicrobial I Panel). This panel was responsible for evaluating data on the active ingredients in this drug class.1 The panel prepared a report to the Commissioner of the FDA classifying OTC drug products into three categories: 1) Category I: generally regarded as safe and effective (GRASE) for the claimed therapeutic indication; 2) Category II: not GRASE or having unacceptable indications; and 3) Category III: insufficient data available to permit final classification (Table 1). The Antimicrobial I Panel employed seven specific product definitions, namely, 1) skin antiseptic, 2) patient preoperative skin preparation, 3) surgical hand scrub, 4) healthcare personnel handwash, 5) skin wound cleanser; 6) skin wound protectant, and 7) antimicrobial soap (Table 2).
On January 6, 1978, the FDA published a tentative final monograph (TFM) describing the conclusions made by the Commissioner on the safety and efficacy requirements for OTC healthcare antiseptics for healthcare professional use. The report included reclassification of active ingredients and modification of in vitro and in vivo erficacy testing requirements.2
On June 17, 1994, the FDA published a notice of proposed rulemaking in the form of an amended TFM that would establish conditions under which OTC topical healthcare antiseptics are GRASE and not misbranded.6 This notice amended the 1978 TFM to establish a group of drug products for healthcare professional use to include healthcare personnel handwash, surgical hand scrub, and patient preoperative skin preparation. A second group of products primarily used by consumers for 'first aid antiseptics' was published in a separate monograph and included antimicrobials used as skin antiseptics, skin wound cleansers, and skin wound protectants.7 Two other drug products, antiseptic handwashes and hand sanitizers or dips, will be addressed in separate monographs. Antiseptic handwashes are used by consumers for personal use in the home such as when changing diapers, after assisting ill persons, and before contact with a person under medical care or treatment. Hand sanitizers or dips are also used by food handlers in federally inspected meat and poultry processing plants and in food handling establishments such as restaurants. The 1994 rulemaking included definitions for antiseptic drugs, broad spectrum activity, and healthcare antiseptics. It also revised definitions for healthcare personnel handwash, surgical hand scrub, and patient preoperative skin preparation to reflect FDA's proposed effectiveness criteria (Table 3).
FDA REGULATORY APPROVAL PROCESS
When developing an antiseptic drug product, there are two options a manufacturer can pursue: the new drug application (NDA) process or the over-the-counter (OTC) drug review known as the monograph system. Table 4 shows the legal distinctions between NDA-direct-OTC and OTC drug monographs and the explicit differences in regulatory approaches for product approval.8,9 NDAs are defined by law as being recognized as safe and effective (RASE). A new chemical entity never before marketed in the United States would be classified as a new drug and in most cases, initially approved for prescription use only. The approved NDA is manufacturer-specific and allows only that particular sponsor to market the product. Other manufacturers wanting to market a similar product would also need to seek FDA approval through an NDA. FDA considers a drug safe enough to approve when the benefits outweigh the risks. This risk-to-benefit assessment is critical in the drug approval process.
OTC drugs are defined as GRASE for their intended use provided they are not misbranded nor marketed using false or misleading statements. A manufacturer desiring to market a monographed (therapeutic classes of ingredients that are GRASE) drug need not seek clearance from the FDA prior to marketing. In this case, marketing is not exclusive and all data and information supporting GRASE status are publicly available. Monographs mainly address active ingredients in the product, and in most cases, final formulations are not subject to monograph specifications. Manufacturers are free to include any inactive ingredients that serve a pharmaceutical purpose, provided those ingredients are considered safe and do not interfere with product effectiveness or required final product testing. In some instances, even though the product may contain GRASE ingredients, the final formulation may need to meet a monograph testing procedure. An example would be the antiseptic drug products that are for healthcare personnel handwash, surgical hand scrub, and patient preoperative skin preparation. These are required to meet in vivo and in vitro efficacy testing requirements to ensure that their formulated products are effective as an antiseptic. Inactive ingredients and emollients, when included in the products, may inhibit the antiseptic action, therefore testing must be performed to show effectiveness. Because the drugs in the monograph system are GRASE, there has been no requirement to report adverse events.
The OTC drug review is a three-phase rulemaking process allowing public comment, with each phase requiring publication in the FR. The FR is a daily publication in which federal agencies publicly announce their regulations and legal notices. The first phase of the OTC drug review is accomplished by FDA-appointed advisory review panels. These panels are assigned by therapeutic categories to review the appropriate ingredients and labeling of marketed OTC drug products to determine whether they could be classified as GRASE. Their recommendations are published and a comment period follows. The second phase describes the FDA's evaluation of the panels' finding, consideration of public comment, and study of any new data, and is published as a tentative final monograph (TFM). This is also followed by a comment period. After considering new data and comments, the FDA issues a final rule which is the third phase of the rulemaking process. Once an OTC drug monograph is finalized, any new conditions that are not included in the final regulations, i.e., ingredients, combinations of ingredients, indications, and labeling can be petitioned to be included or to go through the NDA route. If an active ingredient is not included in the final rulemaking, manufacturers have two separate approaches to gain marketing clearance. They may either submit supportive data in the form of a petition to amend a final monograph to include the new marketing conditions or submit an NDA for OTC drug use.8,9 Table 5 provides a brief overview on the status and characteristics of currently marketed antiseptic agents.
COMMON ANTISEPTICS
Alcohol
There are three types of alcohol used on the skin: ethyl alcohol (ethanol), isopropyl alcohol (used in the U.S.), and normal-propyl alcohol (n-propyl, used in Europe).10 The latest TFM for healthcare antiseptic drug products (1994 FR) establishes ethanol (60% to 95%) as Category I, safe and effective for healthcare personnel handwash, surgical hand scrub, and patient preoperative skin preparation (for preparation of the skin prior to injection). Isopropyl alcohol (50% to 91.3%) is recognized as Category I, safe and effective for patient preoperative skin preparation (for preparation of the skin prior to injection). These alcohols have excellent in vitro bactericidal activity against most gram-positive and gramnegative bacteria. They also kill Mycobacterium tuberculosis, various fungi, and certain enveloped viruses; however, they are not sporicidal and have poor activity against certain nonenveloped viruses.4,10 Because of their lack of sporicidal action, alcohols are not recommended for sterilizing surgical tools and instruments.
The alcohol killing mechanism appears to stem from protein coagulation and denaturation. Kamm reported associated alcohol-mediated disruptions of cytoplasmic integrity, cell lysis, and interference with cellular metabolism.12 Protein coagulation occurs within certain concentration limits.
The presence of water plays an important role in the anti-microbial activity of alcohol. The most concentrated forms of ethanol (100%) and isopropyl alcohol (100%) are less bactericidal than mixtures of alcohol and water because proteins are not denatured readily in the absence of water.11 Dry bacterial cells are more resistant to bactericidal action than moist bacterial cells.13 It has been reported that 70% and 50% ethanol killed Mycobacterium tuberculosis more rapidly than 95% when the organism was suspended in water or sputum.14
Isopropyl alcohol is slightly more toxic than ethanol. There are cases of toxic reactions reported in children after sponge bathing with isopropyl alcohol to reduce fevers. The vapors may be absorbed through the lungs, and cases of acute poisoning by this means have occurred.15,16,17 Overall, isopropyl alcohol is considered safe and effective for use as an OTC topical antimicrobial agent.2,11 Adverse effects include skin irritation and dryness. Recently marketed preparations add emollients to minimize skin drying. Studies have shown that emollients may also enhance antimicrobial activity.11,18-21
Alcohols are rapidly bactericidal and fast acting but not considered persistent. The regrowth of bacteria begins soon after use of alcohol based antiseptics.22 Persistent activity can be achieved by addition of an active ingredient such as clorhexidine. This combination not only provides a strong immediate effect, but also a continuing, antibacterial effect that is desirable for use as a surgical hand disinfectant and a patient preoperative skin preparation.18,23 Alcohol-based hand rubs for healthcare professionals in hospitals are available as foams, rinses, and gels. Currently, limited data are available regarding efficacy of these types of alcohol formulations. Alcohol-based hand rubs should not replace sinks because when hands are visibly soiled or contaminated with proteins or organic matter such as blood or other body fluids, they must be washed with soap and water. Alcohols are highly flammable and volatile and must be properly stored. To prevent risk of operating room fire, preoperative skin preparation solutions containing alcohol should be allowed to completely dry before use of a laser or electrocautery.24,25 The use of alcohol antiseptics has been around since the 1930s and there have been no reports of alcohol-resistant bacteria strains.26,27
Iodine
Tincture of iodine, containing approximately 2% iodine, has been long used as a preoperative skin preparation. Tincture of iodine causes some stinging and irritation.28,29 However, based on the risk-benefit ratio, the one-time use is justified. It is considered safe and fast acting; nevertheless, the use is limited to painting of the operative site prior to surgery and must be removed with 70% alcohol immediately after drying to avoid potential skin irritation.2,3
Iodophors are the most common form of topical iodine. Iodophors depend on the release of free iodine as the active agent. The complexing molecule acts only as a carrier. Iodophors increase the solubility of iodine and allow for sustained-release. Iodophors have a reduced equilibrium concentration of free iodine with increased antimicrobial efficacy.28 The most common iodophor is povidone-iodine, a complex using 1-vinyl-2-pyrrolidinone polymer. According to the United States Pharmacopeia (USP) XXIII, povidone iodine contains no less than 9% and no more than 12% available iodine. Introduced in the 1960s, povidone-iodine contains a low amount of free molecular iodine, reducing toxic effects, staining, and irritation.29 Povidone-iodine provides slow and continuous release of free iodine. Free iodine degrades microbial cell walls and cytoplasm, denatures enzyme, and coagulates chromosomal material.
The iodine released when the complex is in contact with the skin is not only available to kill microorganisms, but is also adsorbed by dead skin cells or other organic material. The killing spectrum of iodines and iodophors is broad and includes gram positive and gram negative bacteria, fungi, viruses, and protozoa.28-30 However, iodophors are less active against certain fungi and spores than are iodine tinctures.31 The antimicrobial activity of iodophors is affected by pH, temperature, exposure time, concentration of total available iodine, and concentration of emollients. Also, iodophors are rapidly neutralized in the presence of organic material such as blood or sputum.29,30
Povidone iodine absorption has been a concern in the treatment of pregnant and lactating mothers because of the possibility of induced transient hypothyroidism.32-34 Fetuses, premature infants, and low birth weight (
Chlorhexidine gluconate
Chlorhexidine gluconate (CHG) has been used for more than 30 years in the clinical setting. In 1976, the FDA granted approval of CHG for use as a topical antiseptic based on its high level of antimicrobial activity, low toxicity, and strong affinity for binding to the skin and mucous membranes. CHG was not an OTC drug monograph active ingredient at that time. CHG disrupts the microbial cell membrane and precipitates the cell contents. CHG (0.5% to 4%) is more effective against gram-positive than gram-negative bacteria and has less activity against fungi and tubercle bacilli. CHG is inactive against bacteria spores, except at elevated temperatures.40,41 Lipid-enveloped viruses, e.g., herpes virus, HIV, respiratory viruses, influenza virus, and cytomegalo-virus are rapidly inactivated. Non-enveloped viruses, e.g., rotavirus, adenovirus, and enteroviruses are not inactivated by exposure to CHG.40,42,43 Numerous studies indicate that CHG is safe and nontoxic.42 It is not absorbed through the skin and has a low skin-irritancy potential. However, severe skin reactions may occur in infants less than two months of age. The potential for allergic contact sensitization, and photosensitization is reported to be minimal. However, CHG should not come into contact with eyes, the middle ear, or meninges.42 Although not as rapidly effective as the alcohols, a major attribute of CHG is its persistence, as it binds the skin and remains active for at least six hours. Although it is not significantly affected by organic matter such as blood, it is pH-dependent, and hence the formulation significantly affects activity. The optimum range of 5.5 to 7.0 corresponds to the pH of body surfaces and tissues.
CHG is used extensively for disinfection of surgeons' and nurses' hands, and provides whole body disinfection of patients undergoing surgery. Low concentration (0.5% to 1%) CHG is added to alcohol-based preparations to provide greater residual activity than alcohol alone. The immediate bactericidal action of CHG surpasses antiseptic preparations containing povidone-iodine, triclosan, hexachlorophene, or chloroxylenol. Its persistence, which prevents regrowth of microorganisms on the skin, is comparable to that of hexachlorophene or triclosan. CHG has a broader spectrum of activity than the others especially against gram-negative bacteria.42 Bacterial resistance to CHG has not been reported, but overgrowth by naturally resistant gram-negative bacteria may sometimes occur.
Hexachlorophene
Hexachlorophene is primarily effective against gram-positive bacteria. It is a chlorinated bisphenol that interrupts bacterial electron transport, inhibits membrane bound enzymes at low concentrations, and ruptures bacterial membranes at high concentrations.44,45 Three percent hexachlorophene kills gram-positive bacteria within 15 to 30 seconds, but a longer time is needed for gram-negative bacteria. Hexachlorophene has residual activity for several hours after application and has a cumulative effect after multiple uses.46,47 Hexachlorophene has been associated with severe toxic effects, including deaths. It can be absorbed through damaged skin of adults and the skin of premature infants.48 Baby powder accidentally contaminated with 6% hexachlorophene has caused infant deaths. The FDA published a final order in 37 FR 20160, September 27, 1972 making 3% hexachlorophene available only by prescription and designating it as unsafe for OTC distribution. Concentrations less than 0.1% may be used.. This concentration is used to preserve cosmetics.48
Hexachlorophene is indicated to control outbreaks of grampositive infections. Hexachlorophene is indicated as a bacteriostatic skin cleanser for surgical scrubbing or handwashing as part of patient care. It is also used as a topical application to control outbreaks of gram-positive organisms when other infection control procedures have been unsuccessful. Hexachlorophene should be used only as long as necessary for infection control. Studies have shown that 1% chlorhexidine powder is at least as effective as hexachlorophene for topical use to control staphylococcal infection in the neonatal setting.49,50 There is evidence for hexachlorophene resistance in plasmids of Pseudomonas aeruginosa.51
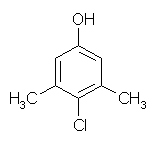
Chloroxylenol
Chloroxylenol (PCMX) is a halogen-substituted phenolic compound that has been used in the U.S. since the 1940s.52 PCMX at concentrations of 0.5% to 4.0% acts by microbial cell wall disruption and enzyme inactivation.26 PCMX has good activity against gram-positive bacteria, but it is less active against gram-negative bacteria, Mycobacterium tuberculosis, fungi, and viruses. The antimicrobial activity of PCMX is unaffected by organic materials such as blood or sputum, but it is neutralized by nonionic surfactants and polyethylene glycol. It is considered intermediate to slow acting and has minimal persistent effect of over a few hours. PCMX has low antimicrobial efficacy compared to iodines, iodophors, and CHG in reducing skin flora.53,54 The FDA classified PCMX (0.24% to 375%) as Category I for safety and Category III for effectiveness for short-term use such as patient preoperative skin preparation. PCMX is classified as Category III for safety and effectiveness for long-term uses, i.e., healthcare personnel handwash and surgical hand scrub.7 The FDA is currently evaluating the safety and efficacy of PCMX for use as a healthcare antiseptic under the OTC drug review. PCMX is currently on the OTC market in concentrations of 0.3% to 3.75%.
Triclosan
Triclosan is a diphenyl ether that disrupts the cell wall. The reaction time is intermediate while the persistence is excellent. It has good activity against gram-positive bacteria, gram-negative bacteria, and viruses. It has fair activity against Mycobacterium tuberculosis and poor activity against fungi. Triclosan is not significantly affected by organic matter such as blood, but is affected by pH and the presence of surfactants and emollients, and hence formulation significantly affects activity.55,56 Triclosan can be absorbed through intact skin but appears to be nonallergenic and nonmutagenic with short-term use.1 The FDA classified triclosan as Category I for safety and Category III for effectiveness for short-term use such as patient preoperative skin preparation. It is classified as Category III for safety and effectiveness for long-term repeat uses for healthcare personnel handwash and surgical hand scrub.7
Safety and efficacy evaluation of triclosan for use as a healthcare antiseptic is currently underway at the FDA. Triclosan has been incorporated into a variety of many personal care products, including toothpastes, deodorant soaps, underarm deodorants, shower gels, and healthcare personnel handwashes. The development of in vitro triclosan bacteria resistance occurs by target mutations, enzymatic modification, and active efflux.57 Currently, there is no evidence of resistance seen in hospital clinical isolates and it poses no risk to healthcare personnel in a real life situation such as in a medical setting.
CONCLUSION
There are numerous antimicrobial agents currently being evaluated by FDA for Healthcare professional use.7 It is important that, when determining the use of antimicrobial agents for healthcare personnel handwash, surgical hand scrub, and patient preoperative skin preparation, the desired characteristics are considered in evaluating evidence of the product's safety and efficacy. Although there is some laboratory evidence regarding emerging antimicrobial resistance in antiseptic products, there is currently no clinical evidence to suggest that topical antiseptic usage in healthcare and medical facilities at their current levels compromises the effectiveness of healthcare hygiene procedures.
REFERENCES
1. Food and Drug Administration. OTC Topical Antimicrobial Products. Tentative Final Monograph Topical Antimicrobial Drug Products, Federal Register, January 17 1978;43:1210-49.
2. Price PB. The bacteriology of normal skin; a new quantitative test applied to a study of the bacterial flora and the disinfectant action of mechanical cleansing. J Infect Dis 1938;63:301-18.
3. Crabtree TD, Pelletier SJ, Pruett TL. Surgical antisepsis. In: Block SS. editor. Disinfection, sterilization, and preservation. 5th ed. Philadelphia: L Williams and Wilkins; 2001. 919-34.
4. Boyce JM, Pitter D. Guideline for hand hygiene in health-care settings: recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/ SHEA/ PIC/ IDSA Hand Hygiene Task Force. Infect Control Hosp Epidemiol 2002;23(12 Suppl):S3-40.
5. Food and Drug Administration. OTC topical antimicrobial products and cosmetic products. Notice of proposed rulemaking, Federal Register, September 13 1974;39:33102-41.
6. Food and Drug Administration. OTC topical antimicrobial products. Tentative final monograph for healthcare antiseptic drug products, Federal Register, June 17 1994;59:31402-52.
7. Food and Drug Administration. OTC topical antimicrobial products. Tentative final monograph for first aid antiseptic drug products, Federal Register, June 17 1991;56:33644-80.
8. Gilbertson WE. FDA's review of OTC drugs. In: Handbook of nonprescription drugs, 10th ed. Washington DC: American Pharmaceutical Association 1993. p 21-37.
9. Sheldon AT. Food and Drug Administration perspective on topical antiseptic drug product development. In: Paulson DS, editor. Handbook of topical antimicrobials. New York: Marcel Dekker Inc; 2003. p 19-48.
10. Morton HE. The relationship of concentration and germicidal efficiency of ethyl alcohol. Ann NY Acad Sci 1950;53:191-6.
11. Ali Y, Dolan MJ, Fendler EJ, Larson EL. Alcohols. In: Block SS, editor. Disinfection, sterilization, and preservation. 5th ed. Philadelphia: L Williams and Wilkins; 2001. p 229-53.
12. Kamm O. The relation between structure and physiological action of alcohols. J Amer Pharm Assoc 1921;10:87-92.
13. Rotter ME. Alcohols for antisepsis of hands and skin. In: Ascenzi JM, editor. Handbook of disinfectants and antiseptic. New York: Marcel Dekker Inc; 1996. p 177-233.
14. Smith CR. Alcohol as a disinfectant against the tubercle bacillus. Public Health Rep 1947;62:1285-95.
15. Wise JR. Alcohol sponge baths. New Engl J Med 1969:280:840.
16. Senz EH, Goldfarb. Coma in a child following use of isopropyl alcohol in sponging. J Pediatr 1958:53:322-3.
17. McFadden SW, Haddow JE. Coma produced by topical application of isopropanol. J Pediatr 1969;43:622-3.
18. Eowbury EJE, Eilly HA, Ayliffe GA. Preoperative disinfection of surgeon's hands: use of alcoholic solutions and effects of gloves on skin flora. Br Med J 1974;4:369.
19. Earson EE, Eke PI, Eaughon BE. Efficacy of alcohol-based hand rinses under frequent-use conditions. Antimicrob Agents Chemother 1986;30:542-4.
20. Ncwman JL, Seitz JC. Intermittent use of an antimicrobial hand gel for reducing soap-induced irritation of health care personnel. Am J Infect Control 1990;18:194-200.
21. Rotter ML, Koller W, Neumann R. The influence of cosmetic additives on the acceptability of alcohol-based hand disinfectants. J Hosp Infect 1991;18(suppl B):57-63.
22. Lilly HA, Lowbury EJE, Wilkins MD, Zaggy A. Delay antimicrobial effects of skin disinfection by alcohol. J Hyg 1979;82:497-500.
23. Rotter ME. Povidone-iodine and chlorhexidine gluconate containing detergents for disinfection of hands [letter to the editor]. J Hosp Infect 1981;2:273.
24. Brown TP, Mills SM, Muller MJ. Burns sustained from alcohol based skin preparations. ANZJ Surg 2003;73:550-1.
25. Tooher R, Maddern GJ, Simpson J. Surgical fires and alcohol-based skin preparations. ANZ J Surg 2004;74:382-5.
26. McDonnell GM, Russell AD. Antiseptics and disinfectants: activity, action, and resistance. Clin Microbiol Rev 1999;Jan:147-79.
27. Jones RD. Bacterial resistance and topical anti-microbial wash products. AmJ Infect Control 1999;27:351-63.
28. Bloomfield SF. Chlorine and iodine formulations. In: Ascenzi JM, editor. Handbook of disinfectants and antiseptics. New York: Marcel Dekker Inc; 1996. p 133-58.
29. Gottardi W. Iodine and iodine compounds. In: Block SS, editor. Disinfection, sterilization, and preservation. 5th ed. Philadelphia: E Williams and Wilkins; 2001. p 159-84.
30. Zamora JE. Chemical and microbiologie characteristics and toxicity of povidone-iodine solutions. Am J Surg 1986;151:400-6.
31. Rutala WA. APIC guidelines for selection and use of disinfectants. AmJ Infect Control 1995;23:313-42.
32. Bryant WP, Zimmerman D. Iodine-induced hyperthyroidism in a newborn. Pediatrics 1995;95:434-6.
33. KogaY, SanoH, Kilkukawa Y, and others. Effect on neonatal thyroid function of povidone-iodine used on mothers during perinatal period. J Obstet Gynaecol 1995;21:581-5.
34. Casteels K, Punt S, Bramswig J. Transient neonatal hypothyroidism during breastfeeding after post-natal maternal topical iodine treatment. Eur J Pediatr 2000;159:716-7.
35. Lin CP, Chen W, Wu KW. Povidone-iodine cord care interferes with neonatal screening for hypothyroidism. Eur J Pediatr 1994;153:756-8.
36. Gordon CM, Rowitch DH, Mitchell ML, Kohane IS. Topical iodine and neonatal hypothyroidism. Arch Pediatr Adolesc Med 1995;149:1336-9.
37. Linder N, Davidovitch N, Reichman B, and others. Topical iodine-containing antiseptics and subclinical hypothyroidism in preterm infants. J Pediatr 1997;131:434-9.
38. Brown RS, Bloomfield S, Bednarek FJ, and others. Routine skin cleansing with povidone-iodine is not a common cause of transient neonatal hypothyroidism in North America: a prospective controlled study. Thyroid 1997;7:395-400.
39. Houang ET, Gilmore OI, Reid C, Shaw EI. Absence of bacterial resistance to povidone iodine. J Clin Pathol 1976;29:752-5.
40. Ranganathan NS. Chlorhexidine. In: Ascenzi JM, editor. Handbook of disinfectants and antiseptic. New York: Marcel Dekker Inc; 1996. p 235-64.
41. Shaker LA, Russell AD, Furr JR. Mechanism of resistance of Bacillus subtilis spores to chlorhexidine. Int J Pharm 1986;34:51-6.
42. Demon GW. Chlorhexidine. In: Block SS, editor. Disinfection, sterilization, and preservation. 5th ed. Philadelphia: L Williams and Wilkins; 2001. 321-36.
43. Springthrope VS, Grenier JL, Lloyd-Evans N, Sattar SA. Chemical disinfection of human rotaviruses: efficacy of commercially-available products in suspension tests. J Hyg 986;97:139-61.
44. Joswick HL, Corner TR, Silvernale JN, Gerhardt P. Antimicrobial actions of hexachlorophene: release of cytoplasmic materials. J Bacteriol 1971;108:492-500.
45. Silvernale JN, Joswick HL, Corner TR, Gerhardt P. Antimicrobial actions of hexachlorophene: cytological manifestations. J Bacteriol 1971;108:482-91.
46. Lowury EJL, Lilly HA, Bull JP. Disinfection of hands: removal of resident bacteria. Br Med J 1963;1:1251-61.
47. Kundsin RB, Walter CW The surgical scrub-practical consideration. Arch Surg 1973;107:75-7.
48. Food and Drug Administration. Statements of general policy or interpretation. Hexachlorophene as a component in drug and cosmetic products for human use: Final Rule, Federal Register, September 27, 1972;37:20160-4.
49. Alder VG, Burman D, Simpson RA, and others. Comparison of hexachlorophene and chlorhexidine powders in prevention of neonatal infection. Arch Dis Child 1980;55:277-80.
50. Wilcox MH, Hall J, Gill AB, and others. Effectiveness of topical chlorhexidine powder as an alternative to hexachlorophene for the control of Staphylococcus aureus in neonates. J Hosp Infect 2004;56:156-9.
51. Sutton L, Jacoby GA. Plasmid-determined resistance to hexachlorophene in Pseudomonas aeruginosa. Antimicrob Agents Chemother 1978;13:634-6.
52. Bruch M. Chloroxylenol: an old-new antimicrobial. In: Ascenzi JM, editor. Handbook of disinfectants and antiseptic. New York: Marcel Dekker Inc; 1996. p 265-94.
53. Sheena AZ, Stiles ME. Efficacy of germicidal hand wash agents in hygienic hand disinfection. J Food Protect 1982;45:713-20.
54. Aly R, Maibach HI. Comparative antibacterial efficacy of a 2-minute sutgical scrub with chlorhexidine gluconate, povidone-iodine, and chloroxylenol sponge brushes. Am J Infect Control 1988;16:173-7.
55. Goddard PA, McCue KA. Phenolic compounds. In: Block SS, editor. Disinfection, sterilization, and preservation. 5th ed. Philadelphia: L Williams and Wilkins; 2001. p 255-81.
56. Marzulli FN, Bruch M. Antibacterial soaps: benefits versus risks. In: Maibach HI, Aly R. editors. Skin microbiology: relevance to clinical infection. New York: Springer-Verlag; 1981. p 125-34.
57. Poole K. Mechanisms of bacterial biocide and antibiotic resistance. Symp Ser Soc Appl Microbiol. 2002;31:55-64
Michelle M Jackson PhD is a Microbiologist at the Food and Drug Administration at Rockville, MD.
Address for correspondence: Michelle M Jackson PhD, Microbiologist, Food and Drug Administration (FDA), Center for Drug Evaluation and Research, Office of Nonprescription Products, 5600 Fishers Lane, HFD-560, Rockville MD 20857. (301) 827-2284. jacksonm@cder.fda.gov
Connie Mahon MS CLS is the Focus: Antimicrobial Resistance guest editor.
Focus Continuing Education Credit: see pages 188 to 191 for learning objectives, test questions, and application form.
Michelle Jackson is an employee of the U.S. Food and Drug Administration and as such has no financial holdings and receives no financial support from any manufacturers. The views and opinions expressed in this publication are the author's and not necessarily those of either the Center for Drug Evaluation and Research or the U.S. Food and Drug Administration.
Copyright American Society for Clinical Laboratory Science Summer 2005
Provided by ProQuest Information and Learning Company. All rights Reserved