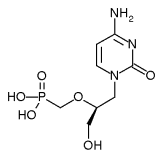
A new drug is available for treating cytomegalovirus (CMV) retinitis, a potentially severe eye infection that can lead to blindness.
While other therapies must be given daily, the new intravenous treatment, Vistide (cidofovir), is administered once a week for the first two weeks and then once every two weeks.
FDA based its June 26 approval on two studies in a total of 148 patients. One study showed that the retinitis progressed more slowly in patients treated immediately with Vistide than in patients with delayed treatment. The second study showed the drug was effective in patients with relapsing CMV retinitis who had received other therapy previously.
Vistide's most significant side effect is potential kidney damage, which can be reduced with the drug Benemid (probenecid) and by hydrating the body to increase its water content. Other side effects included decreased white blood cells, weakness, nausea, diarrhea, and decreased pressure in the eye.
Some studies have shown that the drug may cause cancer in rats, but such cancers have not been seen in human studies.
Vistide is manufactured by Gilead Sciences Inc., of Foster City, Calif.
(See also "Living with Aids: New Treatments Give Hope," in the January-February 1992 FDA Consumer.)
COPYRIGHT 1996 U.S. Government Printing Office
COPYRIGHT 2004 Gale Group