Study objectives: To demonstrate equivalence in the clinical efficacy of telithromycin vs clarithromycin treatment of outpatients with acute exacerbations of chronic bronchitis (AECB), and to compare the tolerability and respiratory-related health-care resource utilization associated with these treatment regimens.
Design and patients: A randomized, double-blind, multicenter, clinical study was conducted at 105 centers in 14 countries. Adult outpatients (age [greater than or equal to] 30 years) received oral telithromycin, 800 mg qd for 5 days (n = 270), or oral clarithromycin, 500 mg bid for 10 days (n = 282), for the treatment of AECB. Clinical and bacteriologic outcomes were assessed at the posttherapy/test-of-cure (TOC) visit (days 17 to 24; per-protocol population). Health-care resource utilization data were collected for each patient by investigators blinded to study medication up to the late posttherapy visit (days 31 to 36).
Results: Clinical cure rates at the posttherapy/TOC visit were comparable between the groups (telithromycin, 193 of 225 patients [85.8%]; clarithromycin, 206 of 231 patients [89.2%]); bacteriologic outcome was satisfactory for 59 of 72 telithromycin-treated patients (81.9%) vs 63 of 76 clarithromycin-treated patients (82.9%). Health-care resource utilization assessed up to the late posttherapy visit was lower in the telithromycin treatment group than the clarithromycin treatment group, with significantly fewer hospitalizations for respiratory-related causes (one hospitalization vs eight hospitalizations for a total of 4 inpatient days vs 39 inpatient days, respectively), significantly fewer AECB-related emergency department visits (0 vs 8), and fewer unscheduled outpatient visits (11 vs 18). Fewer telithromycin-treated patients reported days lost from work (21 of 91 patients [23.1%]; 133 days) compared with those receiving clarithromycin (30 of 98 patients [30.6%]; 141 days). Telithromycin was well tolerated; adverse events considered possibly related to study medication were reported by 61 of 269 patients (22.7%) and 100 of 280 patients (35.7%) receiving telithromycin and clarithromycin, respectively. Conclusions: In this study, 5-day telithromycin treatment was as effective and well tolerated as 10-day clarithromycin treatment for patients with AECB, and was associated with a reduced utilization of health-care resources.
Key words: acute exacerbations of chronic bronchitis; clarithromycin; health-care utilization; ketolide; telithromycin
Abbreviations: AE = adverse event; AECB = acute exacerbations of chronic bronchitis; CI = confidence interval; CNALV = clinically noteworthy abnormal laboratory value; ED = emergency department; mITT = modified intent to treat; PP = per protocol; PPb = bacteriologic per protocol; PPc = clinical per protocol; RTI = respiratory tract infection; TEAE = treatment-emergent adverse event; TOC = test of cure; ULN = upper limit of normal
**********
Acute exacerbations of chronic bronchitis (AECB) are a common and costly problem. The prevalence of chronic bronchitis is estimated at between 3% and 17% in developed countries. (1) Subjects with chronic bronchitis are predisposed to frequent exacerbations (one to four episodes per year), characterized by increased dyspnea, sputum production, and sputum purulence, (2) which may lead to a progressive deterioration of respiratory function,a
Results from placebo-controlled clinical investigations (2,4,5) have demonstrated the clinical efficacy of antibacterial agents in the treatment of AECB, particularly for those patients with at least two of the three symptoms of AECB (worsening dyspnea, increased sputum volume, and increased sputum purulence) and/or severe airway obstruction. The acquisition of new strains of bacterial pathogens has been linked with episodes of AECB, (6) and the most common bacterial pathogens causative of AECB--accounting for up to 70% of AECB infections--include Haemophilus influenzae, Streptococcus pneumoniae, and Moraxella catarrhalis. (1) While their role is controversial, atypical/ intracellular pathogens including Chlamydophila (Chlamydia) pneumoniae may account for another 10 to 15% of cases. (7,8) Patients with AECB are usually treated empirically with orally administered antibacterial agents, including macrolides/azalides, [beta]-lactams, and fluoroquinolones. However, antibacterial resistance among the key respiratory pathogens (9-13) threatens the use of these agents in the treatment of AECB.
Total health-care costs associated with AECB in the United States were estimated at $1.7 billion in 1994, (14) with hospitalizations (resulting from initial treatment failure) accounting for the greatest proportion of the total cost of management of AECB. (14-16) The failure of antibacterial therapy to markedly reduce or eliminate causative pathogens of AECB may contribute to treatment failure; therefore, the continued development of new antibacterials that provide coverage of the key respiratory pathogens is important.
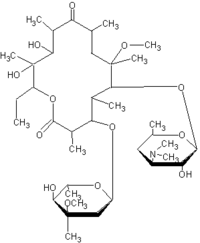
Telithromycin, a ketolide antibacterial structurally related to the macrolides, has a spectrum of activity targeted against common respiratory tract infections (RTIs), including both typical (S pneumoniae, H influenzae, and M catarrhalis) and atypical respiratory pathogens causative of AECB, and strains resistant to penicillin and/or macrolides. (17-21) Telithromytin also demonstrates a low potential for the selection or induction of macrolide-lincosamidestreptogramin B resistance, (22,23) Pharmacokinetic studies (24,25) have indicated that a regimen of 800 mg qd of telithromycin provides concentrations in plasma and bronchopulmonary tissue adequate to maintain activity against respiratory pathogens.
The aim of this study was to demonstrate equivalence in clinical efficacy and to assess the safety of a 5-day course of oral telithromycin, 800 mg qd, vs a standard 10-day course of oral clarithromycin, 500 mg bid, in the treatment of adult patients with AECB. Health-care resource utilization was also assessed prospectively throughout the study by investigators blinded to study treatment.
MATERIALS AND METHODS
This randomized, double-blind, parallel-group study took place at 105 centers in 14 countries. The study was performed in accordance with Good Clinical Practice guidelines and was approved by the independent ethics committee of each center. All patients provided written informed consent prior to enrollment.
Study Subjects
Adult outpatients (aged [greater than or equal to] 30 years) with a documented history of chronic bronchitis, characterized by cough and excessive sputum production for [greater than or equal to] 2 consecutive years and most days in a consecutive 3-month period, and a clinical diagnosis of AECB presumably due to bacterial infection based on all of the three criteria of Anthonisen et al (2) (dyspnea, increased sputum volume, and increased sputum purulence) were eligible for inclusion.
Patients were required to have negative chest radiograph findings for acute pulmonary infiltrates within 2 days prior to initiation of study medication. Sputum specimens for Gram stain and bacteriologic culture were also collected at inclusion. Patients were excluded from the study if they presented with acute bronchitis, asthma, lung cancer or metastases, or other conditions increasing the likelihood of nonbacterial infection: bronchiectasis, cystic fibrosis, or active pulmonary tuberculosis. Those patients requiring aggressive airway management, those with acute respiratory failure, or patients hospitalized for parenteral antibiotic therapy were also excluded. Other criteria for exclusion included a known or suspected hypersensitivity to macrolide antibacterials, or a requirement for concomitant treatment with medications known to have adverse interactions with either study drug (including ergot alkaloid derivatives, terfenadine, cisapride, astemizole, pimozide, and quinidine). Patients who had received clarithromycin for this infectious episode, or those who had received azithromycin, dirithromycin, or ceftriaxone or other antibiotics for > 24 h in the 7 days prior to enrollment, were also excluded.
Study Design and Medication
On entry to the study (day 1), patients underwent a physical examination. Medical history was documented, including assessment of respiratory function in the past 12 months; number of acute exacerbations in the past year; history of documented bronchial obstruction; and bronchodilator and oxygen use and corticosteroid therapy within the past year. Infection-related signs and symptoms were assessed. The peak expiratory flow rate was measured, blood samples were collected for clinical laboratory assessment, and chest radiography and 12-lead ECG were performed.
Sputum samples were collected within 48 h before initiation of therapy for the culture, isolation, identification, and antibacterial susceptibility testing of causative pathogen(s). Only those samples containing [greater than or equal to] 25 polymorphonuclear cells and [less than or equal to] 10 squamous cells per low-power magnification (x 100) were considered appropriate for culture. Investigators assessed all isolated pathogens to determine whether they were causative of the current infection. Susceptibility testing was performed locally using disk diffusion methods according to the National Committee for Clinical Laboratory Standards methodology. All isolated organisms that were considered to be "causative pathogens" were shipped to a central reference laboratory (Clinical Microbiology Institute, Wilsonville, OR) for reidentification and determination of the minimum inhibitory concentration.
Patients were randomized (1:1) to receive oral treatment with either telithromycin, 800 mg qd plus placebo qd for 5 days, followed by matched placebo bid for 5 days, or clarithromycin, 500 mg hid for 10 days. The identity of the treatment regimen was blinded by encapsulating active medication and placebo in opaque capsules. Clinic visits were scheduled for days 3 to 5 (on-therapy visit), days 11 to 13 (end-of-therapy visit), days 17 to 24 (posttherapy/test-of-cure [TOC] visit), and days 31 to 45 (late posttherapy visit). Infection-related signs and symptoms, overall clinical status, respiratory function tests (FE[V.sub.1]/FVC), safety/ tolerability, and health-care resource utilization (including outpatient visits, emergency department [ED] visits, and hospitalizations) were assessed at all visits. Additionally, at each visit sputum samples were obtained (where possible) for bacteriologic analysis, and blood samples were collected for culture from febrile patients when clinically warranted. In the event of clinical deterioration or failure, a chest radiograph was performed and reviewed by the investigator and subsequently read and results documented by a certified radiologist. Clinical laboratory assessments (chemistry and hematology) were performed on blood samples collected at the on-therapy and end-of-therapy visits (and at any subsequent visit if clinically significant abnormal results were recorded).
Efficacy Analysis
The primary objective of this study was to assess clinical outcome at the posttherapy/TOC visit. Patients were considered to be clinically cured if, in the opinion of the investigator, the AECB-related signs and symptoms had disappeared or returned to the preinfection state or the remaining AECB-related signs and symptoms represented a normal clearance of infection without the need for additional antibacterial therapy. Clinical failures included those patients with the following: all AECB-related signs and symptoms remained unchanged or worsened, there was insufficient improvement and new antibiotics or subsequent treatment were added, there were new clinical findings consistent with active infection, or a new antibiotic was required because of adverse events (AEs) associated with study treatment that led to discontinuation, nonadherence, or bacterial persistence. If circumstances precluded classification as cure or failure, the clinical outcome was classified as indeterminate.
Secondary efficacy analyses included bacteriologic outcome at the posttherapy/TOC and late posttherapy visits, and clinical outcome at the last posttherapy visit. Bacteriologic outcome was assessed at the posttherapy/TOC visit and classified as satisfactory if the causative pathogen was absent (eradication) or the patient had improved clinically to such an extent that a follow-up culture could not be obtained (presumed eradication). An unsatisfactory outcome was recorded if the causative pathogen was still present (persistence) or was presumed persistent (eg, if there was a need for new antibacterial therapy due to clinical failure). Other criteria for classification as an unsatisfactory bacteriologic outcome included emergence of a new pathogen during therapy (superinfection), reappearance of the causative baseline pathogen after eradication from the original site of infection (recurrence), or appearance of a new bacterial strain with the presence of signs/symptoms of infection (reinfection).
Health Outcome Analysis
At each of the scheduled study visits, a health outcome questionnaire designed to evaluate health-care resource utilization associated with AECB was completed for all patients. Unscheduled AECB-related outpatient visits, AECB-related ED visits, time lost from work due to AECB, and hospitalizations evaluated by the investigator as being AECB related were captured prospectively throughout the study on ease report forms. All non-AECB-related hospitalizations recorded by the investigators on serious AE forms were subsequently evaluated by a physician blinded to study treatment. These hospitalizations were categorized as either "other respiratory related" (ie, non-AECB-related) or "nonrespiratory related."
Safety and Tolerability
All patients who received at least one dose of study medication with one posthaseline safety assessment were eligible for inclusion in the safety analysis. All AEs spontaneously reported by patients and those observed by investigators were recorded and assessed in terms of causality (possibly related or not related) and severity. Safety and tolerability were also assessed based on measurements of laboratory safety parameters and ECG recordings performed at baseline, during treatment, and on completion of treatment. Laboratory safety variables (including hematology and serum chemistry parameters) were assessed to identify potentially abnormal values using predefined extended normal ranges (comprising extended normal ranges adapted for subjects with infectious diseases to minimize the selection of abnormal values due to the course of the infection). Clinically noteworthy abnormal laboratory values (CNALVs) were those values considered medically important by the study sponsor according to predefined criteria and included alanine transaminase > 3 times the upper limit of normal (ULN) range, aspartate transaminase > 3 times the ULN range, alkaline phosphatase > 1.5 times ULN range, and bilirubin > 2 times the ULN range.
Statistical Analysis
All randomized subjects who received at least one dose of study medication and with signs and symptoms of AECB were included in the modified intent-to-treat (mITT) population. All mITT patients excluding those with major protocol violations were eligible for the per-protocol clinical (PPc) analysis. Bacteriologic efficacy was assessed in the per-protocol bacteriologic (PPb) population, which consisted of those subjects in the PPc population with isolation of a causative pathogen from an adequate pretherapy culture specimen obtained within 48 h of initiation of therapy.
The primary efficacy analysis was to demonstrate equivalence between the two treatment groups for clinical cure rates at the TOC visit in the PPc population. A two-sided 95% confidence interval (CI) for the difference in cure rates between the two groups was calculated, and equivalence was concluded if the lower limit of the 95% CI for the treatment difference was [greater than or equal to] - 15% and the upper limit crossed zero. (26,27)
The statistical analysis plan for health outcome data was finalized prior to unblinding of the database. Tests performed on these data included Fisher Exact Test for measures of incidence and the Wilcoxon rank-sum test for continuous measures. All treatment-emergent adverse events (TEAEs) and safety/tolerability laboratory variables were summarized using descriptive statistics.
RESULTS
A total of 650 patients from 14 countries were enrolled into the study between March 14, 2001, and January 11, 2002 (Argentina, n = 28; Australia/New Zealand, n = 44; Belgium, n = 24; Brazil, n = 5; Canada, n = 54; Chile, n = 21; Germany, n = 82; Italy, n = 16; Mexico, n = 18; South Africa, n = 72; Spain, n = 4; Turkey, n = 18; United States, n = 264). Of these, 554 patients were randomized to receive treatment with either telithromycin, 800 mg qd for 5 days (n = 272), or clarithromycin, 500 mg bid for 10 days (n = 282). Two patients were subsequently excluded from the telithromycin group because of confirmed pneumonia; therefore, the mITT population comprised a total of 552 patients (telithromycin, n = 270; clarithromycin, n = 282).
A total of 96 patients (45 from the telithromycin group and 51 from the clarithromycin group) were excluded from the PPc analysis due to one or more major protocol violations, the most common being insufficient duration of treatment, age 30 years, insufficient AECB infection-related signs at pretherapy/entry, missing appropriate posttreatment information, and inability to determine clinical outcome at the posttherapy/TOC visit. Baseline demographics and disease characteristics of the PPc population and the mITT population were comparable, with no major between-group differences (Table 1). In the mITT population, few patients (6 of 270 patients receiving telithromycin and 5 of 282 patients receiving clarithromycin) had received prior treatment with other antibacterials (permitted for up to 24 h within the 7 days prior to study enrollment). Relevant concomitant illnesses were recorded as general risk factors for morbidity and were similar across treatment groups in the mITT and PPc populations (Table 1). The most common of these illnesses were chronic airway obstruction (telithromycin treatment group, 70.4% [190 of 270 patients]; clarithromycin treatment group, 70.2% [198 of 282 patients]) and coronary atherosclerosis (telithromycin treatment group, 8.5% [23 of 270 patients]; clarithromycin treatment group, 7.8% [22 of 282 patients]) [mITT population]. Use of the most common concomitant nonantibacterial medications was balanced between the mITT treatment groups: antiasthmatic agents were received by 59.6% (161 of 270 telithromycin patients) and 60.6% (171 of 282 clarithromycin patients), and systemic corticosteroids were received by 17.4% (47 of 270 of telithromycin patients) and 23.8% (67 of 282 clarithromycin patients).
Adherence to treatment regimens was comparable for the two groups, with 88.1% of telithromycin-treated patients and 87.6% of clarithromycin-treated patients achieving 100% adherence over the 10-day treatment period (which included a 5-day placebo treatment period for telithromycin-treated patients). Over the first 5 days of treatment, 91.5% of telithromycin-treated patients and 92.2% of clarithromycin-treated patients achieved 100% adherence.
Clinical Outcomes
Analysis of the pooled PPc population revealed that 5 days of treatment with telithromycin yielded efficacy results equivalent to 10 days of treatment with clarithromycin (Table 2). At the posttherapy/ TOC visit, 85.8% of patients treated with telithromycin were clinically cured vs 89.2% of those treated with clarithromycin (difference, -3.4; 95% CI, - 9.3 to 3.1). Cure rates in the mITT population at the posttherapy/TOC visit were also equivalent between the groups: 83.0% with telithromycin vs 83.7% with comparator (Table 2). Per-protocol (PP) cure rates at the late posttherapy assessment reflected a low level of relapse in both groups (Table 2), with equivalent efficacy between the treatment groups: 79.8% with telithromycin vs 82.0% with comparator (difference, - 2.2; 95% CI, - 10.2 to 5.7).
Analysis of clinical outcomes was performed on subgroups with demographic features and current infection characteristics of special interest. In the telithromycin treatment group, rates of clinical cure for patients aged [greater than or equal to] 65 years, those with severe AECB (as assessed by the investigator), and those who smoked were 83.0% (78 of 94 patients), 85.2% (23 of 27 patients), and 86.2% (81 of 94 patients), respectively. The respective cure rates for clarithromycin-treated patients were 88.1% (89 of 101 patients), 82.6% (19 of 23 patients), and 87.5% (77 of 88 patients). A difference in clinical cure rate was noted in the subgroup of patients with significant airway obstruction (ie, FE[V.sub.1]/FVC < 60%) at the posttherapy/TOC visit: 61 of 81 patients (75.3%) in the telithromycin group compared with 65 of 76 patients (85.5%) in the clarithromycin group. However, this difference diminished by the late posttherapy visit, with clinical cure rates of 55 of 78 patients (70.5%) and 50 of 67 patients (74.6%) in the telithromycin and clarithromycin groups, respectively.
Bacteriologic Outcomes
At baseline, a total of 177 pathogens determined to be causative of AECB were isolated from 148 patients in the PPb population, the most common being H influenzae, M catarrhalis, and S pneumoniae (40.1%, 20.9%, and 11.3% of isolates, respectively). Bacteriologic outcomes among the PPb population at the posttherapy/TOC visit were satisfactory for 59 of 72 patients (81.9%) who received telithromycin and 63 of 76 patients (82.9%) treated with clarithromycin. The overall eradication rates (documented eradication and presumed eradication on the basis of clinical results) for the telithromycin and clarithromycin treatment groups were 70 of 86 patients (81.4%) and 78 of 91 patients (85.7%), respectively. Bacteriologic eradication rates were comparable for the three most common causative pathogens isolated in this study: H influenzae, 27 of 35 patients (77.1%) vs 30 of 36 patients (83.3%); M catarrhalis, 17 of 19 patients (89.5%) vs 17 of 18 patients (94.4%); S pneumoniae, 10 of 13 patients (76.9%) and 7 of 7 patients (100%), for telithromycin and clarithromycin, respectively.
Of the eight telithromycin-treated patients with H influenzae who recorded an unsatisfactory bacteriologic response at the posttherapy/TOC visit, three were classified as failures based on documented persistence of the causative pathogen (the remaining five patients included four cases of presumed persistence and one of recurrence). Two of the three patients with documented persistence of H influenzae had baseline risk factors that may have contributed to the clinical outcome of failure, including a history of systemic corticosteroid use, chronic bronchodilator use, and long-term oxygen therapy. Similarly, of the three telithromycin-treated patients with S pneumoniae infection who were classified as bacteriologic failures, all had baseline risk factors and significant underlying diseases that may have contributed to the clinical outcome of failure. For two of these patients, the bacteriologic outcome was presumed persistence, as S pneumoniae was not isolated from subsequent sputum samples; in the remaining patient, S pneumoniae infection was classified as recurrent.
Health-Care Utilization and Time Lost From Work
Hospitalization data were documented by study investigators blinded to treatment group. In the mITT population (comprising patients with a confirmed diagnosis of AECB who received at least one dose of study medication), significantly fewer telithromycin-treated patients were hospitalized for any reason compared with patients receiving clarithromycin (4 patients vs 14 patients, respectively; p = 0.03). One telithromycin-treated patient and four patients receiving clarithromycin were hospitalized due to AECB (p = 0.37) for 4 days and 16 days, respectively. Four clarithromycin-treated patients were hospitalized for other respiratory-related reasons (including increased shortness of breath [n = 1]; pneumonia [n = 1]; pneumonia, dyspnea, and acute renal failure [n = 1]; and respiratory distress [n = 1]) for a combined total of 23 inpatient days, while no patients were hospitalized for other respiratory-related reasons in the telithromycin treatment group. Overall, significantly fewer telithromycin-treated patients were hospitalized for respiratory-related reasons compared with those receiving clarithromycin (one vs eight patients, respectively; p = 0.04) [Fig 1].
Health-care utilization attributed to AECB is summarized in Table 3 for all mITT subjects. The number of AECB-related ED visits was significantly lower in the telithromycin group vs the clarithromycin group (zero patients vs eight patients, respectively; p = 0.008). Eleven telithromycin-treated patients vs 18 clarithromycin-treated patients (p = 0.25) attended unscheduled AECB-related outpatient visits. In total, 12 telithromycin-treated patients and 22 clarithromycin-treated patients recorded AECB-related utilization of health-care resources during the study.
Approximately 40% of patients in each treatment group were > 65 years of age (Table 1), contributing to the fact that the majority of patients were not employed at study entry (telithromycin, 179 of 270 patients [66.3%]; clarithromycin, 182 of 280 patients [65.0%]). Among those patients who were employed, 21 telithromycin-treated patients (23.1%) reported a total of 133 days lost from work due to AECB, compared with 141 days lost by 30 clarithromycin-treated patients (30.6%) [Table 3].
Safety and Tolerability
A total of 549 patients were evaluable for safety (telithromycin, n = 269; clarithromycin, n = 280). Overall, 48.1% (264 of 549 patients) reported at least one TEAE (telithromycin, n = 119 [44.2%]; clarithromycin, n = 145 [51.8%]). TEAEs were most commonly reported for the GI system, affecting 53 telithromycin patients (19.7%) and 72 clarithromycin patients (25.7%). The most common GI TEAEs were diarrhea (telithromycin, n = 19 [7.1%]; clarithromycin, n = 32 [11.4%]) and nausea (telithromycin, n = 14 [5.2%]; clarithromycin, n = 22 [7.9%]).
TEAEs assessed by the investigator to be possibly related to study medication affected 161 patients (29.3%): telithromycin, n = 61 (22.7%); clarithromycin, n = 100 (35.7%). The most common possibly treatment-related TEAEs (those reported by [greater than or equal to] 1% of patients) are shown in Table 4. TEAEs were generally of mild-to-moderate intensity. TEAEs categorized by the investigator as severe occurred in 14 clarithromycin-treated patients (5.0%) and 6 telithromycin-treated patients (2.2%). The frequency of discontinuation of treatment as a result of TEAEs was comparable between the groups (telithromycin, 6 of 269 patients [2.2 %]; clarithromycin, 9 of 280 patients [3.2%]), with diarrhea given as the most common reason for discontinuation.
Fourteen patients experienced serious TEAEs (telithromycin, n = 5 [1.9%]; clarithromycin, n = 9 [3.2%]). One serious TEAE in each treatment group was assessed as possibly related to study medication: a 51-year-old, telithromycin-treated white woman experienced an increase in AECB-related signs and symptoms on day 15 that resolved without sequelae following subsequent antimicrobial treatment; in the clarithromycin-treatment group, a 75-year-old white man experienced an event of coagulation disorder on day 10 (elevated international normalized ratio for prothrombin time) that was reported as an overdose of warfarin. The event resolved following hospitalization and appropriate treatment.
CNALVs were infrequent and comparable between the treatment groups (telithromycin, 43 of 269 patients [16.0%]; clarithromycin, 39 of 280 patients [13.9%]). Nine patients had CNALVs for liver function tests (telithromycin, eight patients; clarithromycin, one patient); however, all nine patients had pretherapy/entry values above the extended normal ranges, and for three of the patients the transaminase CNALV decreased from the pretherapy/entry values. None of these CNALVs led to discontinuation of the study medication.
The change in median QTc value (by the Bazett method (28)) from pretherapy/entry to on-therapy visits was minimal and similar among the treatment groups (telithromycin, 3.7 ms; clarithromycin, 4.7 ms), and no clinically noteworthy ECG QTc values (> 500 ms) were observed in either treatment group. Vital sign changes from pretherapy/entry visits were small and comparable between the treatment groups.
DISCUSSION
In this study, a 5-day treatment course of telithromycin, 800 mg qd, was as effective and as well tolerated as a 10-day course of clarithromycin, 500 mg bid, for the treatment of AECB in adult patients. Furthermore, in a prospective, blinded evaluation of health-care resource utilization, telithromycin treatment was associated with significantly fewer RTI-related hospitalizations (defined as "AECB-related" plus "other respiratory-related" hospitalizations), significantly fewer AECB-related ED visits, fewer AECB-related hospitalizations, and a shorter length of hospital stay compared with clarithromycin treatment.
Clarithromycin at the recommended dosage of 500 mg bid was chosen as the comparator treatment in this study, given its use as a first-line empiric treatment for RTIs. The results of the clinical outcome efficacy analysis at both the posttherapy/TOC visit and the late posttherapy visit demonstrated equivalence between the two treatment groups. The clinical cure rates of 85.8% for telithromycin and 89.2% for clarithromycin at posttherapy/TOC visits were comparable with rates previously reported following treatment of AECB with various antibacterial agents: 84% for ceftibuten, (29) 82% for amoxicillin-clavulanate, (30) 88% for azithromycin, (31) and 89% for moxifloxacin. (32)
The cure rate observed for telithromycin in this study has been confirmed in other comparative clinical trials for the treatment of AECB, with the 5-day course of telithromycin shown to be as effective as a 10-day course of either amoxicillin-clavulanate (30) or cefuroxime axetil. (33) The analysis of clinical cure in subgroups of PP patients, including those aged [greater than or equal to] 65 years, smokers, those with severe symptoms, or with airway obstruction at the late posttherapy visit, indicated that both treatments were similarly efficacious for patients considered to be at increased risk of morbidity/mortality.
Bacteriologic outcome was satisfactory (documented and presumed eradication) for 81.9% of telithromycin-treated patients with pathogens isolated at baseline in the PP population at the posttherapy/TOC visit and comparable with clarithromycin-treated patients (82.9%). Consistent with the observed clinical cure rates, the overall bacteriologic eradication rates (by isolated causative pathogen) for the telithromycin and clarithromycin treatment groups were 81.4% and 85.7%, respectively, and were comparable with previously reported eradication rates. (30,34)
Pharmacoeconomic evaluations of antibacterial use in AECB have identified treatment failures--particularly those resulting in hospitalization--as a major factor contributing to the overall cost of management of AECB. (14-16) A prospective analysis of health-care resource utilization in this study indicated that treatment with telithromycin was associated with lower overall use of health-care resources than clarithromycin treatment, including significantly fewer RTI-related hospitalizations and a shorter length of hospital stay, and significantly fewer AECB-related ED visits, translating into significant potential cost savings. This trial was powered to assess differences in clinical efficacy rather than health-care resource utilization; therefore, the results of these secondary prospective analyses should be regarded as preliminary. Nevertheless, the trend towards a reduction in health-care resource utilization for patients treated with telithromycin was consistent across all health outcomes assessed in this analysis.
Administration of telithromycin for 5 days was generally well tolerated and comparable with clarithromycin treatment for 10 days. The majority of AEs were mild to moderate in intensity, and were generally associated with the GI system (diarrhea, nausea, and dysgeusia). No AEs were ascribed to unexpected drug interactions.
In conclusion, short-course telithromycin, 800 mg qd for 5 days, appears to be as effective and well tolerated as clarithromycin, 500 mg bid for 10 days, in the treatment of AECB in adult patients. These findings, combined with the potential health-care resource cost savings associated with telithromycin treatment, suggest that telithromycin may be an attractive first-line therapeutic option for the management of AECB in outpatients.
This study was sponsored by sanofi-aventis, USA.
Dr. Mandell has received funding from Aventis Bayer, Ortho-McNeil, Pfizer, and Wyeth. Dr. Nusrat is employed by sanofi-aventis, and Dr. Chang and Dr. Rangaraju are former employees of sanofi-aventis. Dr. Fogarty has received funding from Astra-Zeneca, Aventis, Bayer, Boehringer, GlaxoSmithKline, Lilly, Ortho-McNeil, Organon, Pfizer, and Sepracor.
Manuscript received February 26, 2004; revision accepted May 18, 2005.
REFERENCES
(1) Ball P, Make B. Acute exacerbations of chronic bronchitis: an international comparison. Chest 1998; 113(suppl):199S-204S
(2) Anthonisen NR, Manfreda J, Warren CP, et al. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann Intern Med 1987; 106:196-204
(3) Ball P. Acute exacerbations of chronic bronchitis: the clinical problem, patient profiles and recommendations (guidelines) for therapy. J Infect Dis Antimicrob Agents 1999; 16:41-48
(4) Saint S, Bent S, Vittinghoff E, et al. Antibiotics in chronic obstructive pulmonary disease exacerbations: a meta-analysis. JAMA 1995; 273:957-960
(5) Allegra L, Blasi F, de Bernardi B, et al. Antibiotic treatment and baseline severity of disease in acute exacerbations of chronic bronchitis: a re-evaluation of previously published data of a placebo-controlled randomized study. Pulm Pharmacol Ther 2001; 14:149-155
(6) Sethi S, Evans N, Grant BJ, et al. New strains of bacteria and exacerbations of chronic obstructive pulmonary disease. N Engl J Med 2002; 347:465-471
(7) Mogulkoc N, Karakurt S, Isalska B, et al. Acute purulent exacerbation of chronic obstructive pulmonary disease and Chlamydia pneumoniae infection. Am J Respir Crit Care Med 1999; 160:349-353
(8) Karnak D, Beng-sun S, Beder S, et al. Chlamydia pneumoniae infection and acute exacerbation of chronic obstructive pulmonary disease (COPD). Respir Med 2001; 95:811-816
(9) Felmingham D, Gruneberg RN. The Mexander Project 1996-1997: latest susceptibility data from this international study of bacterial pathogens from community-acquired lower respiratory tract infections. J Antimicrob Chemother 2000; 45:191-203
(10) Felmingham D. Evolving resistance patterns in community-acquired respiratory tract pathogens: first results from the PROTEKT global surveillance study. Prospective Resistant Organism Tracking and Epidemiology for the Ketolide Telithromycin. J Infect 2002; 44(suppl):3-10
(11) Hoban DJ. Prevalence and characterization of macrolide resistance in clinical isolates of Streptococcus pneumoniae and Streptococcus pyogenes from North America. J Chemother 2002; 14(suppl):25-30
(12) Chen DK, McGeer A, de Azavedo JC, et al. Decreased susceptibility of Streptococcus pneumoniae to fluoroquinolones in Canada. Canadian Bacterial Surveillance Network. N Engl J Med 1999; 341:233-239
(13) Quale J, Landman D, Ravishankar J, et al. Streptococcus pneumoniae, Brooklyn, New York: fluoroquinolone resistance at our doorstep. Emerg Infect Dis 2002; 8:594-597
(14) Niederman MS, McCombs JS, Unger AN, et al. Treatment cost of acute exacerbations of chronic bronchitis. Clin Ther 1999; 21:576-591
(15) McGuire A, Irwin DE, Fenn P, et al. The excess cost of acute exacerbations of chronic bronchitis in patients aged 45 and older in England and Wales. Value Health 2001; 4:370-375
(16) Miravitlles M, Murio C, Guerrero T, et al. Pharmacoeconomic evaluation of acute exacerbations of chronic bronchitis and COPD. Chest 2002; 121:1449-1455
(17) Barry AL, Fuchs PC, Brown SD. Antipneumococcal activities of a ketolide (HMR 3647), a streptogramin (quinupristin-dalfopristin), a macrolide (erythromycin), and a lincosamide (clindamycin). Antimicrob Agents Chemother 1998; 42:94-946
(18) Bebear CM, Renandin H, Bryskier A, et al. Comparative activities of telithromycin (HMR 3647), levofloxacin, and other antimicrobial agents against human mycoplasmas. Antimicrob Agents Chemother 2000; 44:1980-1982
(19) Dubois J, St-Pierre c. In vitro activity of telithromycin, macrolides and quinolones against respiratory tract pathogens [abstract No. 2152]. In: Abstracts of the 40th Interscience Conference on Antimicrobial Agents and Chemotherapy, September 17-20, 2000, Toronto, Canada: American Society for Microbiology, 2000; 178
(20) Pankuch GA, Visalli MA, Jacobs MR, et al. Susceptibilities of penicillin- and erythromycin-susceptible and -resistant pneumococci to HMR 3647 (RU 66647), a new ketolide, compared with susceptibilities to 17 other agents. Antimicrob Agents Chemother 1998; 42:624-630
(21) Roblin PM, Hammerschlag MR. In vitro activity of a new ketolide antibiotic, HMR 3647, against Chlamydia pneumoniae. Antimicrob Agents Chemother 1998; 42:1515-1516
(22) Davies TA, Dewasse BE, Jacobs MR, et al. In vitro development of resistance to telithromycin (HMR 3647), four macrolides, clindamycin, and pristinamycin in Streptococcus pneumoniae. Antimicrob Agents Chemother 2000; 44:414-417
(23) Bonnefoy A, Girard AM, Agouridas C, et al. Ketolides lack inducibility properties of MLS(B) resistance phenotype. J Antimicrob Chemother 1997; 40:85-90
(24) Namour F, Wessels DH, Pascual MH, et al. Pharmacokinetics of the new ketolide telithromycin (HMR 3647) administered in ascending single and multiple doses. Antimicrob Agents Chemother 2001; 45:170-175
(25) Khair OA, Andrews JM, Honeybourne D, et al. Lung concentrations of telithromycin after oral dosing. J Antimicrob Chemother 2001; 47:837-840
(26) Doyle CA, Beltangady M. Statistical methods used in equivalence trials for anti-infective drugs. Proc Biopharm Sect Am Stat Assoc 1993; 392-395
(27) Makuch R, Simon R. Sample size requirements for evaluating a conservative therapy. Cancer Treat Rep 1978; 62:10371040
(28) Bazett HC. An analysis of the time-relations of electrocardiograms. Heart 1920; 7:353-370
(29) McAdoo MA, Rice K, Gordon GR, et al. Comparison of ceftibuten once daily and amoxicillin-clavulanate three times daily in the treatment of acute exacerbations of chronic bronchitis. Clin Ther 1998; 20:88-100
(30) Aubier M, Aldons PM, Leak A, et al. Telithromycin is as effective as amoxicillin/clavulanate in acute exacerbations of chronic bronchitis. Respir Med 2002; 96:862-871
(31) Whitloek W. Multicenter comparison of azithromycin and amoxicillin/clavulanate in the treatment of patients with acute exacerbations of chronic obstructive pulmonary disease. Curr Ther Res 1995; 56:985-995
(32) Wilson R, Kuhin R, Ballin I, et al. Five day moxifloxacin therapy compared with 7 day clarithromycin therapy for the treatment of acute exacerbations of chronic bronchitis. J Antimicrob Chemother 1999; 44:501-513
(33) Zervos MJ, Heyder AM, Leroy B. Oral telithromycin 800 mg once daily for 5 days versus cefuroxime axetil 500 mg twice daily for 10 days in adults with acute exacerbations of chronic bronchitis. J Int Med Res 2003; 31:157-169
(34) Ziering W, McElvaine P. Randomized comparison of once-daily ceftibuten and twice-daily clarithromycin in the treatment of acute exacerbation of chronic bronchitis. Infection 1998; 26:68-75
* From Spartanburg Pharmaceutical Research (Dr. Fogarty), Spartanburg, SC; 10 NHC Health Centre (Dr. de Wet), Johannesburg, South Africa; McMaster University (Dr. Mandell), Hamilton, ON, Canada; sanofi-aventis (Drs. Chang and Nusrat), Bridgewater, NJ; and sanofi-aventis (Dr. Rangaraju), Romainville, France.
Correspondence to: Charles Fogarty, MD, Spartanburg Pharmaceutical Research, 126 Dillon St, Spartanburg, SC 29307; e-mail: cmf@bonetesting.com
COPYRIGHT 2005 American College of Chest Physicians
COPYRIGHT 2005 Gale Group