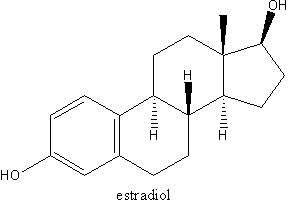
The FDA approved an expanded indication for Climara 0.025 mg, giving it the green light to be marketed as a "starting dose" for the treatment of menopausal symptoms and the prevention of osteoporosis. Berlex Laboratories, the U.S. affiliate of Schering AG, manufacturers the drug. The Climara patch, with its main ingredient being derived from soy, delivers 17-beta estradiol directly through the skin and into the bloodstream for seven days.
Berlex announced separately FDA's approval of Finevin (azelaic acid cream 20 percent) for the topical treatment of mild to moderate inflammatory acne vulgaris. It sells for an average wholesale price of $36.95 and was launched concurrently with a patient education and compliance program.
COPYRIGHT 2001 Lebhar-Friedman, Inc.
COPYRIGHT 2001 Gale Group