Sulfur mustard (HD) is a blister agent targeting the eyes, respiratory system, skin, and possibly other organs. Extensive exposure can destroy the immune system by destruction of bone marrow cells. There is no antidote for HD or effective treatment other than rapid decontamination. Clinical trials have demonstrated activity for N-acetyl-L-cysteine (NAC) against a number of significant human pathologies involving free radicals, and animal and tissue studies have suggested efficacy for NAC as a chemoprotectant against many toxic chemicals. Among these are studies demonstrating that NAC significantly reduces the effects of HD and HD simulants, both in cultured cells and animals. Given the historical effectiveness of HD, the lack of any effective treatment, the demonstrated chemoprotective properties of NAC, its low toxicity, the lack of regulatory controls, and the data supporting efficacy against HD effects, we suggest daily oral administration of the maximum safe dose of NAC to personnel entering combat zones.
The Threat
Sulfur Mustard: History of Use on the Battlefield
Sulfur mustard (bis-(2-chloroethyl) sulfide; CAS 505-60-2) is often referred to by the acronym HD; although HD technically refers to a highly purified form of sulfur mustard generally produced after World War I, in this communication it will be used, for brevity's sake, to refer to any sulfur mustard formulation. HD was first used in war near Ypres, Belgium on July 12, 1917; although it was only used for 1 year, HD was responsible for approximately 80% of the chemical casualties in World War I. Italy used mustard on the Abyssinians (Ethiopians) in its 1935-1936 conquest of that country, and Japan used it in China starting in 1939.1 Mustard was inadvertently released from the SS John Harvey in 1943 by German bombing.2 HD, probably of Soviet make, was used by Egypt in the 1963-1967 Yemen War.3 Iraq used mustard (probably HD) extensively in the Iran-Iraq war,4 causing as many as 45,000 casualties.5
Mustard Effects
HD is a vesicant, i.e., a blister agent. It is an oily liquid of low volatility that causes extensive damage to all contacted tissues. The skin develops erythema typically 4 to 8 hours after exposure. Vesication appears 2 to 18 hours later;6 this delay of effects promotes the spreading of the agent beyond the original deployment site. These blisters will generally coalesce over time to produce large bulk 0.5 to 5 cm in diameter. Liquid mustard may produce a full-thickness (third-degree) burn. Severe skin lesions may require months to heal.7 Inhaled mustard vapor can produce acute airway injury 4 to 6 hours after exposure, including hoarseness, pharyngeal and laryngeal erythema, wheezing and dyspnea, and epithelial sloughing with pseudomembrane formation in severe exposures.6 Initial nonbacterial bronchitis is followed by bacterial superinfection in 4 to 6 days. Ocular exposure to even small amounts of mustard vapor results in irritation; more severe exposures bring on pain, severe conjunctivitis, and corneal damage.6 HD-induced photophobia lasts for weeks. Most deaths result from pulmonary damage complicated by infection and occur several days following exposure. A study of the disease and mortality rate for persons exposed to HD in Saradasht, Iran, in 1987, found that of the 108 deaths that have occurred among severely exposed individuals, 93 died within the first month of HD exposure.8 A majority (85 of 108) of these deaths occurred within the first 2 weeks of exposure and resulted entirely from respiratory failure caused by pulmonary injury or sepsis secondary to infection of the lungs or other tissues. Most who died of respiratory failure died within 2 days of HD exposure. Persons who died of infection and sepsis often developed acute respiratory distress syndrome which resulted in death despite antibiotic therapy.
Two hypotheses exist for the biochemical effects of mustard exposure. HD is a DNA alkylating agent,9 causing strand breaks which are irreparable to cellular machinery.6 The normal response to irreparable DNA damage is apoptosis, sometimes called programmed cell death, in which the cell actively destroys itself through a series of metabolic processes including DNA segmentation, proteolysis of cellular enzymes, and disruption of the cell membrane.10 This is consistent with reports that many HD effects appear to be mediated through apoptotic mechanisms.11-16 Second, because HD reacts rapidly with glutathione (GSH), an intracellular reducing agent involved in many detoxification reactions,17 a second hypothesis for HD effects is the profound depletion of intracellular GSH, leaving the cell susceptible to naturally-produced reactive oxygen species.6 Damage from reactive oxygen species also results in apoptosis.18 It therefore appears that whatever the mechanism, (1) apoptosis is induced as the mechanism of tissue destruction and (2) GSH is a sink for HD damage, reducing the effective dose received by the cell and subsequent cell damage.
Medical Treatment
There is currently no antidote or chemoprophylaxis for HD. Currently, the only means of reducing injury attributable to HD exposure is decontamination of the exposure site within 2 minutes;6 after this time, HD becomes absorbed and conjugated to biomolecules and no benefit is realized from decontamination. Following development of symptoms, only supportive care is available, such as ventilation, antibiotics, and skin burn care.
The Department of Defense Chemical and Biological Defense Program identifies the goal of having a licensed chemoprophylaxis for HD exposure, but not at least until 2009-2010.19 In this article, we provide evidence that suggests that N-acetyl-L-cysteine (NAC) is a candidate chemoprophylaxis material for HD exposure in humans.
N-acetyl-L-cysteine (NAC)
NAC Structure and Metabolism
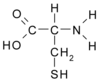
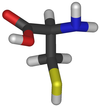
NAC (CAS 616-91-1) is a synthetic cysteine derivative (Fig. 1). Although it has been used in clinical settings for many years (see below), its mechanism of action for observed clinical benefit has been identified much more recently.
GSH is a tripeptide made up of residues of glutamine, glycine, and cysteine (Fig. 2). It is the principle thiol participant in cellular redox reactions and part of the major cellular detoxification pathway; it is involved in the detoxification of most endogenous and exogenous toxicants.20 GSH is also important in the pathology of cancer, acquired immunodeficiency syndrome (AIDS), and other diseases.21 Its principle activities are the reduction of reactive oxygen species (ROS) and free radicals,22 and s-conjugation leading to the increased export of conjugates both from the cell and from the body.23 GSH is synthesized from its component amino acids via γ-glutamyl-cysteine synthase and GSH synthase.24 The limiting factor for this synthesis is the concentration of cysteine, which can be 10- to 20-fold less than the concentration of the other amino acids.24 Therefore, in many cases, the limiting factor in cellular detoxification capacity is the intracellular concentration of cysteine.
NAC can be given orally or intravenously and is readily deacetylated in the liver and intestine to physiological cysteine, whereas administered cysteine tends to auto-oxidize to an insoluble product.24 NAC is absorbed rapidly, if not efficiently, in humans; peak plasma concentration is achieved within 1 to 2 hours after a 200- to 400-mg oral dose. Most of the absorbed NAC is rapidly bound to protein or excreted; reduced NAC has a physiological half-life of 6.25 hours.25 NAC does make an efficient physiological substrate for GSH synthesis: preincubation of cultured hamster cells with NAC prevents depletion of GSH by toxicants.26 In rats, NAC has been demonstrated to increase intracellular GSH in erythrocytes, liver, and lung cells, reduce the activation of procarcinogens by the liver, and detoxify mutagens.27 In humans, oral NAC administration increases GSH levels in T cells of AIDS patients.28 NAC is also able to substitute for GSH as a substrate for microsomal glutathione S-transferase, a major detoxifying enzyme.29 Given the importance of GSH in cellular response to xenobiotics, free radicals, ROS, and other sources of cellular damage, it should be obvious a priori that NAC should relieve or prevent a variety of human pathologies. And there is a large volume of solid experimental evidence that NAC is clinically efficacious as chemical antidote and immune support.
Demonstrated Clinical Benefits
NAC has been used for more than 25 years as an antidote to acetaminophen overdose, both in oral and intravenous form.30 It functions by replenishing and substituting for GSH in detoxifying the hepatotoxic metabolite of acetaminophen, N-acetyl-p-benzoquinonimine. NAC has also been used in nebulized form, as a mucolytic agent, cleaving the sulfhydryl bonds in pulmonary mucus.31 Within the last decade, however, a number of new clinical uses for systemic NAC treatment have been tested in randomized, placebo-controlled, double-blind (RPD) studies.
Human immunodeficiency virus (HIV)-infected patients are deficient in GSH,32 therefore NAC supplementation could potentially help in the treatment of some of the symptoms and conditions in these patients. Several groups have evaluated the efficacy of NAC in the treatment of HlV and AIDS patients. Administration of a daily oral dose of 800 mg of NAC for 4 months normalized plasma cysteine levels, slowed the decline of CD4^sup +^ lymphocytes, and decreased plasma tumor necrosis factor α levels.33 A second study found that NAC administration resulted in a significant improvement of immunological function, if no change in viral load for symptomatic AIDS patients.34 Administration of large oral doses of NAC (median dose 5.3 g/day for a median 24 weeks) was found to restore GSH levels in CD4 and CDS T cells of AIDS patients and improved 2-year survival dramatically.28 However, the reported results were not from a RPD and were potentially confounded by patient self-selection.
There have been several reports of clinical benefits associated with NAC administration to patients with a wide range of serious disease states or injuries. It is not clear at this time whether NAC administration in these patients provides a benefit by positively affecting GSH metabolism/availability or by some mechanistic pathway not yet elucidated.
RPD systemic NAC trials for treatment of acute lung injury showed an improved systemic oxygenation and reduced the need for ventilatory support after 3 days of 40 mg/kg/day of intravenous NAC.35 A similar study with more seriously ill patients36 using 190 mg/kg/day NAC found no change in ventilator need or mortality, but some improvement in lung injury. A 6-month 1,200-mg/day RPD study of 262 subjects, mostly over 65 years old, found that the rate of seroconversion toward A/H^sub 1^N^sub 1^ Singapore 6/86 influenza was similar among study patients regardless of treatment; however, 75% of NAC-treated seroconverters did so asymptomatically, while only 21% of placebo-receiving seroconverters did not show symptoms.37 NAC treatment also improved function of the patients' immune cells in antigen challenge tests. Twenty-six patients with Sjögren's syndrome involved in a RPD crossover study of NAC demonstrated that NAC relieves many of the subjective ocular symptoms, if not the objective laboratory tests.38 NAC increases liver blood flow following septic shock,39 and one recent RPD study40 showed efficacy for NAC in the treatment of cognitive impairment in Alzheimer's disease.
Chemoprotection in Cell Culture and Animal Studies
A broad array of studies have been conducted on the chemoprotective properties of NAC in cultured cells. Catecholamines are natural neurotransmitters used by the central and peripheral nervous systems; however, auto-oxidation produces free radicals which can be cytotoxic to the nerve cells. Of the many antioxidants evaluated, only NAC prevents this catecholamine toxicity in cortical cell cultures.41 Nitric Oxide (NO) donor chemicals, which are used as experimental tools for evaluating the toxic effect of exogenous NO, deplete cell GSH levels, resulting in an increase in apoptotic cell death. Cotreatment of immortalized cells from the human gingival epithelioid S-G cell with NAC + NO donor compounds reduced apoptosis and cytotoxicity as compared with cells not receiving NAC cotreatment.42 Zn^sup 2+^ causes profound morphological changes, production of ROS, and cell death in isolated rat neural cells; addition of NAC to cell culture inhibited the toxic effects of Zn^sup 2+^.43 NAC was shown to significantly inhibit interleukin-8 mRNA and protein levels produced by BET-1A human bronchial epithelial cells following exposure to diesel exhaust; interleukin-8 is a cytokine that promotes apoptosis.44 Acrylamide is tumorigenic in rats and mice and promotes morphological transformation of Syrian hamster embryo cells,26 an indicator of carcinogenic potential. NAC reduces cellular transformation of Syrian hamster embryo below levels occurring in negative controls, even in the presence of a moderate amount of acrylamide. Pretreatment with NAC prevents the destruction of isolated rat testicular germ cells by methoxyacetic acid (a paint industry byproduct), suggesting NAC has the ability to prevent human testicular atrophy, the main hazard of methoxyacetic acid exposure.45
In animals, NAC has been demonstrated to have chemoprotectant properties against a number of hazardous chemicals. NAC given to rats in their drinking water at a dose rate of approximately 1 g/kg/day for 14 days prevented bronchial epithelial thickening/hyperplasia and reduced DNA damage as a result of daily concurrent exposure to cigarette smoke from 25 cigarettes.46 In a similar study, NAC coadministration in drinking water was found to protect rats against various markers of DNA damage as a result of exposure to cigarette smoke for 28 consecutive days.47 NAC moderated the effects of asbestos exposure in rat lungs,48 accelerated healing of free radical-damaged soft tissue,49 and even protected against the lethal effects of perfluoroisobutene exposure in rats.50 It is believed that ROS are important in stimulating programmed cell death in neural cells. Administration of 1% NAC in drinking water to litters of wobbler mice for 9 weeks exhibited a significant reduction in motor neuron loss,51 suggesting that NAC reduced neuron loss by inhibiting ROS-induced cell damage.
Other NAC Activities
Owing to its support of cellular physiology and suppression of apoptosis, NAC also appears to have activity against other types of pathology. NAC enhances the inhibition of prostaglandin synthesis by nonsteroidal anti-inflammatory drugs in isolated monocytes.52 5-Aminolevulinic acid is a product of metabolism which is part of the pathology of porphyria and lead poisoning, accumulating intracellularly and producing free radicals that damage DNA; NAC treatment in cell culture prevented DNA damage.53 NAC given intraperitoneally (163 mg/kg) before or immediately after fluid-percussion brain injury to cats maintained the normal arteriolar constriction response to hyperventilation, whereas control animals lost this response after injury.54 NAC was even able to partially protect mice against challenge with anthrax lethal toxin.55 There is some evidence that NAC administration in mice increases GSH peroxidase enzyme activity which is thought to be important for protecting neural cells from programmed cell death.51 NAC added to cell culture has been shown to induce NF-κB, suggesting that NAC may exert an effect on cell survival-promoting effects independent of its antioxidant properties.51
Mustard Chemoprotection
Although it had been known for some time that HD formed GSH conjugates,56 research in NAC chemoprotection against HD agent began approximately 15 years ago when it was noted that resistance to nitrogen mustard (used as cancer chemotherapy) could be correlated to GSH levels and that depletion of GSH levels would render tumors sensitive to mustard treatment.57 Pretreatment of human peripheral blood lymphocytes with 10 mM NAC reduced the effects of lower concentrations of HD, but not high concentrations, and the suggestion was made at that time that augmentation of intracellular GSH may provide some protection against the HD agent.58 A later study using vascular endothelial cells in a model of capillary leakage and edema11 found that NAC pretreatment nearly eliminated apoptotic effects, although it did little against cellular necrosis. Because most clinical effects of HD appear to be related to apoptosis, this finding was interpreted as supporting the chemoprotective properties of NAC. The same group later found evidence suggesting that the chemoprotective effects of NAC are primarily due to enhanced GSH synthesis.12 Similar experiments using bronchial epithelial cells found NAC cotreatment (NAC administration simultaneous with HD exposure) provided significant, although by no means complete, protection against HD, but pretreatment was not tested.59
NAC cotreatment in rats seemed to virtually eliminate many of the early (≤24 hours) toxic effects associated with HD-induced lung injury.60 Considering that intraperitoneal injection requires time for absorption into the mesenteric capillary system and that HD is typically combined or metabolized within the first few minutes after exposure, this is a significant finding. In a recent study by McClintock et al.,61 they found that NAC reduced lung injury by 70% if administered 10 minutes before HD exposure and had significant benefit if administered up to 90 minutes after exposure.
Combined, the animal and cell data are very promising that NAC supplementation could ameliorate the health effects of HD exposure in humans and thus may benefit military personnel, emergency responders, and other persons with possible high risk for HD exposure. It should be noted, however, that the animal studies described here focus on exposure to relatively low to moderate concentrations of airborne HD; therefore, few conclusions can be drawn about NAC's ability to ameliorate the toxic effects of exposure to high airborne concentrations of HD (e.g., air saturation levels). However, in a typical HD chemical attack similar to ones experienced in World War I and more recent conflicts, the risk of exposure to high airborne concentrations of HD are low. Therefore, persons exposed to HD in a typical attack where airborne concentrations of HD are low to moderate may benefit from even small or moderate protection afforded by NAC supplementation. At this time it is not possible to predict whether NAC supplementation would afford significant protection from dermal toxicity associated with HD exposure. However, to this date studies show that treatment of laboratory animals with NAC increases the levels of GSH in the skin, implying that there may be some benefit or increased protection of the dermis from the toxic effects of HD exposure. Further studies are necessary to determine whether this hypothesis is correct.
NAC Safety
NAC has very low toxicity in humans as demonstrated by its high probable lethal dose of 5 to 15 g/kg body weight,62 or approximately 3.3 pounds for the average person. NAC is commonly packaged as a 600-mg capsule for unregulated sales. As an acetaminophen antidote, NAC is commonly delivered intravenously as a 20% solution, leading to several grams of NAC delivered intravenously within a 15-minute period. Cases of toxicity from this regimen are rare63 and are complicated by symptoms from the overdose.64 No systematic experiments were identified in a Medline/Toxline literature search to determine maximum tolerated dose in humans or in animals.
To answer the question of what may be a reasonable long-term maximum tolerated dose, there are two studies to consider. A study of 262 mostly older (78% ≥ 65 years) who took 1,200 mg/day for 6 months (166 ± 35 days) reported a level of adverse events (9%, mostly mild gastrointestinal effects) not significantly higher than those of the placebo group;37 64 HIV-infected patients took a median dose of 5,300 mg/day for a similar length of time (median 24 weeks)28 and saw no difference between NAC and placebo in the rate of study withdrawal for perceived side effects. To base the human maximum tolerated dose on systemic effects, the latter study would be appropriate; however, delivery was by effervescent tablet containing a large amount of bicarbonate. On the other hand, the participants of both studies were likely to be less resistant to gastrointestinal insult and possibly other negative effects as compared with relatively young and healthy military personnel. Therefore, a dose of 4,800 mg/day (eight standard 600-mg capsules distributed over the day; 90% of the HIV study median dose) probably has a very low risk of significant adverse effects for most persons, with the most likely adverse effects related to NAC intake being nausea or diarrhea. In the event such effects occur, they should be rectifiable by reducing the dose.
Summary and Conclusions
HD attacks mammalian cells by causing DNA lesions which cannot be easily corrected, leading to cell death and, if widespread enough, loss of organ (lung, skin, and immune system) functions. The principle cellular defense against these effects is direct detoxification of HD by GSH conjugation before DNA damage. Consequently, the increase of GSH activity, either through GSH augmentation or by providing a physiologically effective substitute, may be the best means of providing cellular level protection to fielded forces from the effects of sudden HD exposure.
At this time, there is no effective antidote or treatment for HD exposure and a licensed chemoprophylaxis for HD exposure is not expected until at least 2009-2010. A number of proprietary pharmaceutical chemoprotectants are under development, but human trials are still some time off. NAC represents "low-hanging fruit" in this field, which could provide an important stopgap measure until the next generation of protection is available, and should be actively pursued to protect our armed forces personnel. Current data suggest that NAC may provide cost-effective chemoprotection against HD toxicity by a GSH-mediated pathway and its chemoprotective effects extend to many tissues including the liver, lungs, brain, testes, and immune system. This hypothesis is supported by experimental studies in cell culture and animals. Clinical studies have demonstrated the effectiveness of NAC in reducing liver toxicity associated with acetaminophen overdose by a general mechanism involving GSH replenishment. NAC would be expected to reduce HD toxicity in affected tissues by a similar mechanism. There are also several studies that demonstrate a general clinical benefit associated with NAC in patients with a wide range of disease states but it is not known if the mechanism of benefit in these patients is related to GSH-associated pathways.
Despite its positive clinical history, NAC is defined as a dietary supplement and as such is not an investigational new drug and does not fall under the licensure requirements of a prescription medication. There is therefore no legal obstruction to its immediate use to protect combat forces. Conscientious medical practice, however, requires some additional toxicity testing, particularly addressing long-term use by healthy populations (previous long-term studies primarily involved people with compromised health28,37,65) Subchronic tests of NAC toxicity in rodents is ongoing in our laboratory; if warranted following the completion of these studies, voluntary human trials should be performed as soon as possible.
References
1. Smart JK: 1997. History of chemical and biological warfare: an American perspective. In: Medical Aspects of Chemical and Biological Warfare. Washington, DC, TMM Publications, Borden Institute, Walter Reed Army Medical Center, 1997.
2. Alexander SF: Medical Report of the Bari Harbor Mustard Casuallies. Milit Surg 1947; 101: 2-17.
3. Shoham D: Chemical and biological weapons in Egypt. Nonprolif Rev Spring-Summer 1998; 5: 48-58.
4. CIA: Iraq's Weapons of Mass Destruction Programs. Central Intelligence Agency unclassified report. Available at http://www.cia.gov/cla/publlcalions/ iraq_wmd/Iraq_Oct_2002.pdf; accessed October 2002.
5. Cams WS: Chemical Weapons in the Middle East. Washington. DC, The Washington Institute for Near East Policy, 1998.
6. Sidell FR, Urbanetti JS, Smith WJ, Hurst CG: Vesicants. In: Medical Aspects of Chemical and Biological Warfare. Washington, DC, TMM Publications, Borden Institute, Walter Reed Army Medical Center, 1997.
7. Willems JL: Clinical management of mustard gas casualties. Ann Med Milit Belg 1989; 3S:1-61.
8. Ghanei M, Alsani J, Khateri S, Hamadanizadeh K: Public health status of the civil population of Sardasht 15 years following large-scale wartime exposure to sulfur mustard. J Burns 2003; 2: 1-9.
9. Papirmeister B, Gross CL, Meier HL, Petrali JP, Johnson JB: Molecular basis for mustard-induced vesication. Fundam Appl Toxicol 1985; 5: S134-49.
10. Li GM: The role of mismatch repair in DNA damage-induced apoptosis. Oncol Res 1999; 11: 393-400.
11. Dabrwoska MI, Becks LL, Lelli JL Jr, Levee MG, Hinshaw DB: Sulfur mustard induces apoptosis and necrosis in endothelial cells. Toxicol Appl Pharmacol 1996; 141: 568-83.
12. Atkins KB, Lodhi IJ, Hurley LL, Hinshaw DB: N-acetylcysteine and endothelial cell injury by sulfur mustard. J Appl Toxicol 2000; 20: S125-8.
13. Kehe K, Reisinger H, Szinicz L: Sulfur mustard induces apoptosis and necrosis in SCL II cells in vitro. J Appl Toxicol 2000; 20(Suppl l):S81-6.
14. Blaha M, Kohl J, DuBose D, Bowers W Jr, Walker J: Ullrastrudural and histological effects of exposure to CEES or heat in a human epidermal model. In Vitro Mol Toxicol 2001; 14: 15-23.
15. Rosenthal DS, Velena A, Chou FP, et al: Expression of dominant-negative FADD blocks human keratinocyte apoptosis and vesication induced by sulfur mustard. J Biol Chem 2003; 278: 8531-40.
16. Rosenthal DS, Simbulan-Rosenthal CM, Liu WF, et al: PARP determines the mode of cell death in skin fibroblasts, but not keratinocytes, exposed to sulfur mustard. J Invest Dermatol 2001; 117: 1566-73.
17. Gentilhomme E, Neveux Y, Hua A, Thiriot C, Faure M, Thivolet J: Action of bis(β-chloroethyl) sulphide (BCES) on human epidermis reconstituted in culture: morphological alterations and biochemical depletion of glutathione. Toxicol In Vitro 1992; 6: 139-47.
18. Mates JM, Sanchez-Jimenez FM; Role of reactive oxygen species in apoptosis: implications for cancer therapy. Int J Biochem Cell Biol 1999; 32: 157-70.
19. DASDCBD (Deputy Assistant to the Secretary of Defense for Chemical and Biological Defense). 2002. Department of Defense Chemical and Biological Defense Program. Vol I: Annual Report to Congress. Office of the Secretary of Defense. Available e at http://www.acq.osd.mil/cp/nbc02/voll-2002cbdpannualreport.pdf; accessed October 2002.
20. Sies H: Glutathione and its role in cellular functions. Free Radie Biol Med 1999; 27: 916-21.
21. Voehringer DW: Bcl-2 and glutathione: alterations in cellular redox state that regulate apoptosis sensitivity. Free Radie Biol Med 1999; 27: 945-50.
22. Ketterer B: Glutathione S-transferases and prevention of cellular free radical damage. Free Radie Res 1998; 28: 647-58.
23. Keppler D: Export pumps for glutathione S-conjugates. Free Radic Biol Med 1999; 27: 985-91.
24. Griffith OW: Biologic and pharmacologic regulation of mammalian glutathione synthesis. Free Radie Biol Med 1999; 27: 922-35.
25. Holdiness MR: Clinical pharmacokinetics of N-acetylcysteine. Clin Pharmacokinet 1991; 20: 123-34.
26. Park J, Kamendulis LM, Friedman MA, Klaunig JE: Acrylamide-induced cellular transformation. Toxicol Sci 2002; 65: 177-83.
27. DeFlora S, Bennicelli C, Camoiriano A, et al: In vivo effects of N-acetylcysteine on glutathione metabolism and on the biotransformation of carcinogenic and/or mutagenic compounds. Carcinogenesis 1985; 6: 1735-45.
28. DeRosa SC, Zaretsky MD, Dubs JG, et al: N-acetylcysteine replenishes glutathione in HIV infection. Eur J Clin Invest 2000; 30: 915-29.
29. Weinander R, Anderson C, Morgenstern R: Identification of N-acetylcysteine as a new substrate for rat liver microsomal glutathione transferase: a study of thiol ligands. J Biol Chem 1994; 269: 71-6.
30. Smilkslein MJ, Knapp GL, Kulig KW, Rumack BH: Efficacy of oral N-acetylcysteine in the treatment of acetaminophen overdose. N Engl J Med 1988; 319: 1557-62.
31. Kasielski M, Nowak D: Long-lerm administration of N-acetylcysteine decreases hydrogen peroxide exhalation in subjects with chronic obstructive pulmonary disease. Respir Med 2001; 95: 448-56.
32. Buhl R, Holroyd KJ, Mastrangeli A, et al: Systemic glutathione deficiency in symptom free HIV seropositive individuals. Lancet 1989; 334: 1294-8.
33. Åkerlund B, Jarstrand C, Lindeke B, Sönnerborg A, Akerblad A-C, Rasool O: Effect of N-acetylcysteine (NAC) treatment on HIV-1 infection: a double-blind placebo-controlled trial. Eur J Clin Pharmacol 1996; 50: 457-61.
34. Beitkreutz R, Pittack N, Nebe CT, et al: Improvement of immune functions in HIV infection by sulfur supplementation: two randomized trials. J Mol Med 2000; 78: 55-62.
35. Suter PM. Domenighetti G, Schaller MD, Laverriere MC, Ritz R, Perret C: N-acetylcysteine enhances recovery from acute lung injury in man: a randomized, double-blind, placebo-controlled clinical study. Chest 1994; 105: 190-4.
36. Domenighetti G, Suter PM, Schaller MD, Ritz R, Perret C: Treatment with N-acetylcysteine during acute respiratory distress syndrome: a randomized, double-blind, placebo-controlled clinical study. J Crit Care 1997; 12: 177-82.
37. DeFlora S, Grassi C, Carati L: Attenuation of influenza-like symptomatology and improvement of cell-mediated immunity with long-term N-acetylcysteine treatment. Eur Respir J 1997; 10: 1535-41.
38. Walters MT, Rubin CE, Keightly SJ, Ward CD, Cawley MID: A double-blind, cross-over, study of oral N-acetylcysteine in Sjögren's syndrome. Scand J Rheumatol 1986; 61: 252-8.
39. Rank N, Michel C, Haertel C, et al: N-acetylcysteine increases liver blood flow and improves liver function in septic shock patients: results of a prospective, randomized, double-blind study. Crit Care Med 2000; 28: 3799-3807.
40. Adair JC, Knoefel JE, Morgan N: Controlled trial of N-acetylcysteine for patients with probable Alzheimer's disease. Neurology 2001; 57: 1515-7.
41. Noh JS, Kim EY, Rang JS, Kim HR, Oh YJ, Gwag BJ: Neurotoxic and neuroprotective actions of catecholamines in cortical neurons. Exp Neurol 1999; 159: 217-24.
42. Babich H, Zuckerbraun HL: In vitro cytotoxicity of glycol-S-nitrosothiols: a novel class of nitric oxide donors. Toxicol In Vitro 2001; 15: 181-90.
43. Ryu R, Shin Y, Choi JW, et al: Depletion of intracellular glutathione mediates zinc-induced cell death in rat primary astrocytes. Exp Drain Res 2002; 143: 257-63.
44. Abe S, Takizawa H, Sugawara I, Kudoh S: Diesel exhaust (DE)-induced cytokine expression in human bronchial epithelial cells. Am J Respir Cell Mol Biol 2000; 22: 296-303.
45. Rao AVSK, Shaha C: N-acetylcysteine prevents MAA induced male germ cell apoptosis: role of glutathione and cytochrome c. FEES Lett 2002; 527: 133-7.
46. Rogers DF, Jeffery PK: Inhibition by oral N-acetylcysteine of cigarette smoke-induced "bronchitis" in the rat. Exp Lung Res 1986; 10: 267-83.
47. Izzotti A, Balansky RM, D'Agnostini F, et al: Modulation of biomarkers by chemopreventive agents in smoke-exposed rats. Cancer Res 2001; 61: 2472-9.
48. Afaq F, Abidi P, Rahman Q: N-acetyl L-cysteine attenuates oxidant-mediated toxicity induced by chrysotile fibers. Toxicol Lett 2000; 117: 53-60.
49. Van der Laan L, Oyen WJG, Verhofstad AAJ, et al: Soft tissue repair capacity after oxygen-derived free radical-induced damage in one hindlimb of the rat. J Surg Res 1997; 72: 60-9.
50. Lailey AF: Oral JV-acetylcysteine protects against perfluoroisobutene toxicity in rats. Hum Exp Toxicol 1997; 16: 212-16.
51. Henderson J. Javaheri M, Kopko S, Roder JC: Reduction of lower motor neuron degeneration in uwbbtermice by N-acetyl-L-cysteine. J Neurosci 1996; 16: 7574-82.
52. Hoffer E, Baum Y, Nahir AM: N-acetylcysteine enhances the action of anti-inflammatory drugs as suppressors of prostaglandin production in monocytes. Mediators Inflamm 2002; 11: 321-3.
53. Yusof M, Yildiz D, Ercal N: N-acetyl-L-cysteine protects against δ-aminolevulinic acid-induced 8-hydroxydeoxyguanosine formation. Toxicol Lett 1999; 106: 41-7.
54. Ellis EF, Dodson LY, Police RJ: Restoration of cerebrovascular responsiveness to hyperventilation by the oxygen radical scavenger N-acetylcysteine following experimental traumatic brain surgery. J Neurosurg 1991; 75: 774-9.
55. Hanna PC, Kruskal BA, Ezekowitz RA, Bloom BR, Collier RJ: Role of macrophage oxidative burst in the action of anthrax lethal toxin. Mol Med 1994; 1: 7-18.
56. Kinsey VE, Grant WM: Action of mustard gas and other poisons on yeast cells. III. Distribution of fixed mustard gas in yeast. J Cell Comp Physiol 1947; 29: 75-84.
57. Ono K, Shrieve DC: Effect of glutathione depletion by i.-buthione sulfoximine on the cytotoxicity of cyclophosphamide in single and fractionated doses to EMT6/SF mouse tumors and bone marrow. J Natl Cancer Inst 1987; 79: 811-15.
58. Gross CL, Innace JK, Hovatter RC, Meier HL, Smith WJ: Biochemical manipulation of intracellular glutathione levels influences cytotoxicity to isolated lymphocytes by sulfur mustard. Cell Biol Toxicol 1993; 9: 259-67.
59. Rappeneau S, Baeza-Squiban A, Marano F, Clavet J-H. Efficient protection of human bronchial epithelial cells against sulfur and nitrogen mustard cytotoxicity using drug combinations. Toxicol Sci 2000; 58: 153-60.
60. Anderson DR, Byers SL, Vesely KR: Treatment of sulfur mustard (HD)-induced lung Injury. J Appl Toxicol 2000; 20: S129-32.
61. McClintock SD, Till GO, Smith MG, Ward PA: Protection from half-mustard-gas-induced acute lung injury in the rat. J Appl Toxicol 2002; 22: 257-62.
62. Gosselin RE, Smith RP, Hodge HC. Clinical Toxicology of Commercial Products, Ed 5. Williams & Wilkins, Baltimore, 1984.
63. Reynard K, Riley A, Walker BE: Respiratory arrest after N-acetylcysteine for paracetamol overdose. Lancet 1992; 340: 675.
64. Ellenhom MJ, Barceloux DG: 1988. Medical Toxicology-Diagnosis and Treatment of Human Poisoning, p 163. New York, Elsevier Science Publishing Company, 1988.
65. Pendyala L, Creaven PJ: Pharmacokinetic and pharmacodynamic studies of N-acetylcysteine, a potential chemopreventive agent during a Phase I trial. Cancer Epidemiol Biomarkers Prev 1995; 4: 245-51.
Guarantor: LT Andrew J. Bobb, MSC USN
Contributors: LT Andrew J. Bobb, MSC USN; LT Darryl P. Arfsten, MSC USN; CDR Warren W. Jederberg, MSC USN
Copyright Association of Military Surgeons of the United States Jan 2005
Provided by ProQuest Information and Learning Company. All rights Reserved