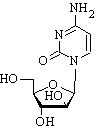
A 44-year-old white male with an isolated central nervous system relapse of acute lymphoblastic leukemia was treated with intrathecal cytarabine. He developed Staphylococcus epidermidis meningitis, which was treated successfully with intrathecal vancomycin. Four weeks after the initiation of intrathecal cytarabine, the patient developed progressive ascending paralysis to the upper cervical level. Initial magnetic resonance imaging of the brain and spine were normal, and cerebrospinal fluid evaluation showed no evidence of ongoing infection and clearance of lymphoblasts. Three weeks later, magnetic resonance imaging demonstrated marked edema and peripheral enhancement of the spinal cord, consistent with cytarabine toxicity.
Introduction
Paraplegia after intrathecal chemotherapy in patients with central nervous system (CNS) leukemia or lymphoma is a rare complication, but it has been recognized.1-7 Cytarabine (cytosine arabinoside, Ara-C; Pharmacia Upjohn, Kalamazoo, Michigan) is a cell cycle-specific agent that has long been used in the prophylaxis and treatment of CNS leukemia. High-dose intravenous administration may cause cerebellar toxicity, peripheral neuropathy, seizures, or encephalopathy. Neurotoxicity from intrathecal (IT) administration is rare but well documented and includes seizures, leukoencephalopathy, or myelopathy.2 Magnetic resonance (MR) imaging findings of spinal cord toxicity have been described rarely in the literature and have emphasized significant abnormalities.2-4 We present a patient in whom quadriplegia developed after intrathecal administration of cytarabine. Initial MR imaging of the spine was normal, but repeat imaging at 3 weeks was diagnostic.
Case Report
A 44-year-old white male had been in his usual state of health until September 1998, when he developed cervical adenopathy. Lymph node and bone marrow biopsies confirmed acute lymphoblastic leukemia (ALL), type LI (pre-B cell lineage). He received induction chemotherapy according to the protocol described by Linker et al.8 and achieved complete remission (aplastic marrow on day 14). He received prophylactic IT methotrexate (12 mg/week for 6 weeks) beginning in November 1998, followed by cranial irradiation at 1800 cGy. In May 1999, the patient developed left eye pain and visual acuity loss. Anterior chamber paracentesis as well as lumbar puncture were positive for relapse of ALL, but bone marrow biopsy was negative. He was treated with electron beam radiotherapy at 600 cGy to the left orbit in July 1999 and was subsequently transferred to our facility for consideration of bone marrow transplantation.
An Ommaya reservoir (Heyer-Schulte Corp., Goleta, California) was placed in the cerebrospinal fluid (CSF) space of the frontal convexity, and treatment with IT cytarabine and hydrocortisone was begun. After five IT treatments, the patient developed Staphylococcus epidermidis meningitis. Treatment with IT vancomycin and intravenous rifampin was initiated, and IT cytarabine was continued. The patient recovered from the meningitis without major neurological sequelae, complaining only of subjective lower extremity weakness. One week after clinical recovery, the patient developed progressive loss of sensorimotor function in the lower extremities and urinary incontinence over a 3- to 4-day period, resulting in complete paralysis below the waist. CSF evaluation at that time was negative for ALL involvement and remained so for the duration of hospitalization. IT therapy was discontinued (total dose of 580 mg of IT cytarabine, 782 mg of IT hydrocortisone, and 12 mg of IT methotrexate), and there was no evidence of ongoing CNS infection.
Initial MR imaging ot the brain and spinal cord was obtained to evaluate the patient's ascending paralysis with a Siemens (Forchheim, Germany) 1.0-Tesla superconducting magnet with standard head and spine coils. The brain and spinal cord were normal (Fig. 1). The patient's myelopathy ascended over a 2-week period to the fourth cervical dermatome. Repeat MR imaging of the spine with contrast was performed 3 weeks after the initial examination. Increased 72-weighted and decreased Tl-weighted signals were seen within the spinal cord, with associated expansion from the tip of the conus to the upper cervical spine (Fig. 2). There was enhancement of the periphery of the cervical and thoracic spinal cord as well as a small focus of enhancement in the midthoracic region (Fig. 3). Repeat MR imaging of the brain remained normal. The patient was quadriplegic but did not require mechanical ventilation. He was transferred to a neurorehabilitation unit for ongoing care in October 1999 and died 3 months later.
Discussion
Since the mid-1960s, IT chemotherapy with methotrexate, cytarabine, or both with or without hydrocortisone has constituted the standard approach to the prophylaxis and treatment of CNS leukemia and lymphoma. IT administration circumvents the blood-brain barrier, minimizes systemic drug exposure and toxic side effects, and allows high drug concentrations at the primary tumor site. The toxicity of CNS therapy rarely involves myelopathy. Patients may present with leg weakness, urinary incontinence, and paresthesias. There is usually a sensory level in the thoracic or lumbar region. The weakness and sensory deficit may ascend, resulting in quadriplegia or death. The syndrome may occur within minutes to as long 6 months after IT therapy.9 The acute syndrome occurring immediately after injection is consistent with a preservative related cause. IT chemotherapy is now given in preservative free solution, and the acute syndrome has not been reported since the cause was recognized.2,5 Hydrocortisone is often administered with IT cytarabine to reduce arachnoiditis and for its potential antileukemic effect. There is no evidence to show that it protects against or contributes to myelopathy.
Watterson et al.2 and Breuer et al.6 first described myelopathy in a small number of patients who were receiving IT cytarabine alone. Direct drug toxicity is likely the primary cause of neurotoxicity observed with IT cytarabine, but why only a small percentage of patients develop this complication is unclear. Collectively, spinal cord toxicity is thought to be related to the cumulative dose intensity of all CNS-directed therapies, possibly as a result of a synergistic effect of the modalities.'2
The neuropathological changes in the spinal cord after IT cytarabine toxicity include demyelination, axonal degeneration, and gliosis of the white matter, predominantly in the periphery of all columns .6 The most severe changes occur in the cervical spinal cord and lateral corticospinal tracts. McLean and colleagues3 described cervical spinal cord MR contrast enhancement that was confined to the lateral columns, whereas Watterson et al.2 described diffuse T2-weighted signal and contrast enhancement of the entire spinal cord. Spinal cord penetration of tritium-labeled cytarabine after IT administration in rabbits is highest in the substantia gelatinosa and the peripheral white matter.10
MR imaging in our patient demonstrated diffusely abnormal spinal cord signal intensity, with expansion and central low signal intensity, predominantly in the cervical region, and predominantly peripheral contrast enhancement. The MR findings are consistent with the pathological findings described by Breuer et al.6 and Burch et al. 10 Central low signal intensity is consistent with edema and neuron loss, Peripheral enhancement corresponds to demyelination, most likely in the regions of the highest drug concentration. Most important is the completely normal MR evaluation of the brain and spinal cord at the onset of our patient's symptoms. There has been one previous report of initially normal MR findings within 1 week of the onset of neurological symptoms secondary to cytarabine and methotrexate toxicity.' Repeat MR imaging was not performed until 6 months later and demonstrated marked cerebellar and spinal cord atrophy on TI-weighted images but no abnormal T2-weighted signal.
The differential diagnosis of ascending paralysis includes acute transverse myelitis, radiation-induced myelopathy, compressive myelopathy, and, rarely, metastatic disease. Exclusion of infection is crucial in the initial evaluation and includes CSF analysis and MR imaging. Imaging findings of myelitis are nonspecific and inelude focal or diffusely increased intramedullary T2-weighted signal. Contrast enhancement can be seen in some cases." Radiation-induced myelopathy is rare and corresponds to the segment of the spinal cord irradiated. Compressive myelopathy must be excluded on initial MR evaluation. MR findings are related to focal cord compression and are easily differentiated from diffuse myelopathy. Metastatic disease of the spinal cord may be epidural, intradural and extramedullary, or intramedullary. Leukemia most commonly presents as leptomeningeal seeding. Postcontrast images demonstrate nodular enhancement along the spinal cord surface and thickened nerve roots.
Conclusion
Transient or permanent paraplegia after IT cytarabine in patients with leukemia or lymphoma is a seldom-described complication. MR imaging plays a crucial role in the initial diagnostic investigation of patients presenting with ascending paralysis. We found a diffusely expanded spinal cord with low Tl-weighted and high T2-weighted signal intensity, suggesting edema and/or necrosis and predominantly peripheral enhancement, which may be secondary to high cytarabine concentrations in the outer spinal cord. Of critical importance is the fact that MR findings were completely normal at the onset of the patient's symptoms. Therefore, in patients who have received IT cytarabine, a normal initial MR scan does not exclude cytarabine toxicity, and a follow-up study is essential. After exclusion of other processes, such as infection or persistent leukemic involvement of the CNS, treatment involves cessation of IT chemotherapy and supportive care.
References
1. Baker WJ, Royer GL, Weiss RB: Cytarabine and neurologic toxicity. J Clin Oncol 1991;9: 679-93.
2. Watterson J, Toogood I, Nieder M, et al: Excessive spinal cord toxicity from intensive central nervous system-directed therapies. Cancer 1994; 74: 303441.
3. McLean DR Clink HK Ernst P, et al: Myelopathy after intrathecal chemotherapy: a case report with unique magnetic resonance imaging changes. Cancer 1994; 73: 3037-40.
4. Lopez-Andreu JA, Ferris J. Verdeguer A, et al: Myelopathy after intrathecal
chemotherapy: a case report with unique magnetic resonance imaging changes Better]. Cancer 1995; 75: 1216-7.
5. Hahn AF, Feasby TE, Gilbert JJ: Paraparesis following intrathecal chemotherapy. Neurology 1983; 33: 1032-8.
6. Breuer AC, Pitman SW, Dawson DM, et al: Paraparesis following intrathecal cytosine arabinoside: a case report with neuropathologic findings. Cancer 1977; 40: 2817-22.
7. Dunton SF, Nitschke R, Spruce WE, et al: Progressive ascending paralysis following administration of intrathecal and intravenous cytosine arabinoside. Cancer 1986; 57: 1083-8.
8. Linker CA, Levit LJ, O'Donnell M, et at: Improved results of treatment of adult acute lymphoblastic leukemia. Blood 1987; 69: 1242-8.
9. Chamberlain MC, Kormanik P. Howell SB, et al: Pharmacokinetics of intralumbar DTIC-101 for the treatment of leptomeningeal metastases. Arch Neurol 1995; 52: 912-7.
10, Burch PA, Grossman SA, Reinhard CS: Spinal cord penetration of intrathecally administered cytarabine and methotrexate: a quantitative autoradiographic study. J Natl Cancer Inst 1988; 80: 1211-6.
11. Gero B, Sze G, Sharif H: MR imaging of intradural inflammatory diseases of the spine. Am J Neuroradiol 1991: 12: 1009-19.
Guarantor: Maj Paul M. Sherman, USAF MC
Contributors: Maj Paul M. Sherman, USAF MC*; MAJ Clifford J. Belden, MC USA^; Capt Douglas A. Nelson, USAF MC*^^
*Department of Radiology and #Department of Hematology/Oncology, Wilford Hall Medical Center, 2200 Bergquist Drive, Suite 1, Lackland Air Force Base, TX 78236-- 5300.
^Department of Radiology, Brooke Army Medical Center, 3851 Roger Brooke Drive, Fort Sam Houston, TX 78234-6200.
Copyright Association of Military Surgeons of the United States Feb 2002
Provided by ProQuest Information and Learning Company. All rights Reserved